Rajasthan Board RBSE Class 11 Chemistry Chapter 1 Basic Concepts of Chemistry
RBSE Class 11 Chemistry Chapter 1 Text Book Questions
RBSE Class 11 Chemistry Chapter 1 Multiple Choice Questions
Question 1.
Number of significant figures in 0.0287 is
(a) 5
(b) 2
(c) 3
(d) 4
Answer.
(c) 3
![]()
Question 2.
Molecular mass of glucose is
(a) 342 u
(b) 110 u
(c) 90 u
(d) 180 u
Answer:
(d)
Question 3.
Volume of 2 g methane at STP will be
(a) 2.8 L
(b) 5.6 L
(c) 11.2 L
(d) 22.4
Answer:
(a) 2.8 L
![]()
Question 4.
At STP, the number of molecules in 1 ml of ideal gas will be
(a) 6.023 × 1023
(b) 2.69 × 1019
(c) 2.69 × 1023
(d) 458 × 1026
Answer:
(b)
Question 5.
Which of the following has least weight?
(a) 108 g of silver
(b) 1 mol of sulphur
(c) 1 g atom of nitrogen
(d) 3.011 × 1023atoms of carbon
Answer:
(d)
RBSE Class 11 Chemistry Chapter 1 Very Short Answer Type Questions
Question 6.
What is the unit and symbol for quantity of matter in SI system?
Answer:
In SI system, the unit and symbol for quantity of matter are mole and mol respectively.
Question 7.
What is the difference between 8.0 g and,8.000 g weight?
Answer:
8.0 g has two significant figures which shows precision up to 1 decimal place where as 8.000 g, has 4 significant figures which shows precision up to 3 decimal place.
![]()
Question 8.
Express 3600 g in three significant figures.
Answer:
3.60 × 103
Question 9.
How many gram molecules of hydrogen are present in 20 g hydrogen?
Answer:
2 g of hydrogen molecules = 1 mol
1 g of hydrogen molecules = 1/2 mol
20 g of hydrogen molecules=1/2 × 20= 10 mol
Question 10.
How many molecules will be present in 64 g oxygen?
Answer:
Gram molecular weight of oxygen = 32 g
32 g of O2 contains = 6.023 × 1023 molecules
Hence, 64 g of O2 will contain 6.023 × 101023 × 64/32
= 6.023 × 1023 × 2
=12.046 × 1023 molecules
![]()
Question 11.
Calculate equivalent weight of H2SO4 Molecular mass of H2SO4 is 98.
Answer:
Equivalent weight of H2SO4 = Molecular mass / number of replaceable H+
= 98/2 = 49
Question 12.
State Avogadro’s law,
Answer:
Avogadro’s law states that “equal volumes of all gases, at the same temperature and pressure, have the same number of molecules”.
Question 13.
How many atoms of carbon will be present in 0.1 mole of C6H12O6.
Answer:
Number of moles of carbon in 1 mol of C6H12O6 = 6
Number of carbon atoms in 1 mole of C6H12O6
= 6 × 6.023× 1023 atoms
Number of carbon atoms is 0.1 mol of C6H12O6
= 0.1 × 6 × 6.023 × 1023 atoms
= 3.6138 × 1023 atoms
![]()
Question 14.
What are significant figures?
Answer:
Each of the digits of a number that are used to express it to the required degree of accuracy or precision, starting from the first non-zero digit, are significant figures.
Question 15.
What is atomicity of a gas?
Answer:
Atomicity of a gas is the number of atoms of an element present in one molecule of that element. For example, helium is monoatomic and hydrogen is diatomic.
Question 16.
Calculate the number of electrons present in 36 g water.
Answer:
Gram molecular weight of H2O= 18 g
According to molecular weight concept
18 g H2O= 6.023 × 1023 molecules of water
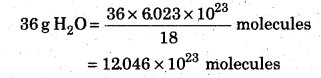
1 molecule of water contains=10 electrons
12.046 × 1023 molecules will contain
=10 × 12.046 × 1023 electrons
=12.046 × 10 24
![]()
Question 17.
What do you understand by molecular mass?
Answer:
Molecular mass is a number equal to the sum of the atomic masses of the atoms in a molecule. The molecular mass gives the mass of a molecule relative to that of the 12 C atom, which is taken to have a mass of 12. Example: molecular mass of H2O = 18.
Question 18.
What is the mass of water which contains 50% heavy water (D2O)?
Answer:
Water containing half H2O and half D2O will have mass = average of masses of,H2O (18) and D2O (20)
Mass of water = (18 + 20)/ 2 = 38/ 2 = 19
Question 19.
What do you understand by standard temperature and pressure?
Answer:
The standard temperature is 273.15 K (0° Celsius or 32° Fahrenheit) and the standard pressure is 1 atm pressure or 760 mm. This is the freezing point of pure water at sea level atmospheric pressure. At STP, one mole of gas occupies 22.4 L of volume (molar volume).
![]()
Question 20.
At STP, x mL N2 gas reacts Completely with xmL O2 gas and forms gas A. What will be the molecular formula of A if volume is unchanged after reaction?
Answer:
According to Avogadro’s Law, equal volume of gases contains equal number of molecules at STP. Therefore number of oxygen and nitrogen molecules will be equal. Hence the formula will be NO.
RBSE Class 11 Chemistry Chapter 1 Short Answer Type Questions
Question 21.
What is the weight of 1 mole of the following
(i) NaCl
(ii) CaCO3
Answer:
(i) Molecular mass of NaCl : 23 amu + 35.5 amu = 58.5 amu
Molar of mass : NaCl: 58.5 g/mol
No. of Moles = Mass / Molar mass
Mass = 1 × 58.5g
Therefore, mass of 1 mole of NaCl is 58.5 g.
(ii) Molecular mass of CaCO3= (1 × Ga + 1 × C + 3 × O)
= (1 × 40 + 1 ×12 + 3 × 16)
= 40 + 12 + 48 = 100
Molar mass of CaCO3= 100 g
Nor of Moles = Mass / Molar mass
Mass = 1 × 100 = 100 g
Therefore, mass of 1 mole of CaCO3 is 100 g.
![]()
Question 22.
Calculate number of significant figures in each of the following:
(i) 0.00468
(ii) 753
Answer:
Both numbers have 3 significant figures.
Question 23.
What will be the mass of 2 moles of following:
(i) MgSO4
(ii) KCl
Answer:
(i) Mass of 1 mole = molar mass
Mass of 1 mole of MgSO4 = 120 g
Mass of 2 moles of MgSO4 = 120 g x 2 = 240 g
(ii)Mass of 1 mole = molar mass
Mass of 1 mole of KCI = 74.5 g
Mass of 2 mole of KCI = 74.5 x 2 = 149 g
Question 24.
Calculate number of significant figures, in each of the following:
(i) 0.868
(ii) 3.865 × 104
Answer:
(i) 3 significant figures
(ii) 4 significant figures
![]()
Question 25.
What is mole? Explain.
Answer:
1 mole is the amount of substance that contains the same number of entities as there are atoms in exactly 12 g of the 12 C isotope.
∴ Number of atoms in 12 g of 12C-isotope
= 6.023 × 1023 atoms/mol
The term ‘mole’ is given by the “Amedeo Avaogadro”. So it is also known as Avogadro’s number (NA).
∴ 1 mole =NA= 6.023 × 1023 atoms, molecules, ions etc.
Question 26.
What is the Limiting Reagent? Explain with the help of example.
Answer:
A limiting reagent is a reactant which is present in lesser amount in a reaction. When it gets consumed, the reaction will not proceed irrespective of the amount of other reactant present in the reaction. It limits the amount of product formed. In other words, it determines the extent of reaction. Let us consider a chemical reaction which is initiated by passing a spark through a reaction vessel containing 10 moles of H2 and 7 moles of O2.
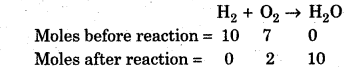
The reaction stops only after consumption of 5 moles of O2 as no further amount of H2 is left to react with
unreacted O2. Thus H2 is a limiting reagent in this reaction.
![]()
Question 27.
Give formula for calculation of equivalent weight of the compound.
Answer:
Equivalent weight of acid = molecular weight of acid/basicity
For example, Equivalent weight of H2SO4 = \(\frac{98}{2}\) = 49 g/eq
Equivalent weight of base = molecular weight of base/acidity
Example, Equivalent weight of NaOH = \(\frac{40}{1}\) = 40 g/eq Equivalent weight of salt = molecular weight of salt/charge present on ionic form ‘
Example, Equivalent weight of Na2CO3= \(\frac{106}{2}\)
= 53 g/eq
Question 28.
Define molarity, molality and normality of a solution.
Answer:
Molarity: It is the number of moles, of the solute dissolved per litre of the solution. It is represented by the symbol ‘M’. It is given as:
M = Mass of solute/Volume of solution in litre.
Unit of Molarity is mol/L.
Molality = It is the number of moles of the solute dissolved in 1 kg of the solvent. It is represented by the symbol ‘m’. It is given as :
m = Moles of solute / Weight of solvent in kg
Unit of molality is mol/kg.
Normality: Normality is number of gram equivalents of the solute dissolved per litre of solution. It is denoted by N.
Normality = No. of gram equivalent of solute / Volume of solution
The unit of Normality is gram equivalent per litre,
![]()
Question 29.
Write the formula of the following compounds and calculate molecular mass :
(i) Calcium Carbonate
(ii) Magnesium Phosphate
(iii) Ferric chloride ,
Answer:
(i) Calcium carbonate = CaCO3.
Molecular mass = (1 x Atomic mass of calcium) + (1 x Atomic mass of carbon) + (3 x atomic mass of oxygen)
= (1 × 40+1 × 12 + 3 ×16) .
= 40 + 12 + 48= 100 g/Aol
(ii) Magnesium Phosphate : Mg3(PO4)2
Molecular mass = (3 x Atomic mass of Mg) + 2 x (1 x atomic mass of P + 4 x atomic mass of 0)
= (3 × 24) + 2[1 × 31 + 4 × 16]
= 72+2(31 + 64)
= 72+2(95)
= 72+190
= 262 g/mol
(iii) Ferric Chloride: FeCl3
= 12.046 × 1023 atoms
Molecular mass = (1 × Atomic mass of Fe + 3 × atomic Now, gram atomic mass of Nitrogen = 14 g mass of Cl) . So, 14 g nitrogen contains 6.023 × 023atoms
= (55.8 + 3 × 35.5)
= (55.8 + 106.5)
= 162.35 g/mol
Question 30.
Calculate the number of moles in the following:
(i) 100 g of CaCO3
(ii) 80 g of Oxygen
(iii) 10 g of C12H22O11
Answer:
(i) Gram molar mass of CaCO3is 100 g.
So, 100 g CaCO3 contains 1 mole of it.
(ii) Gram atomic mass of Oxygen is 16 g.
So, 16 g of Oxygen contains 1 mole of it
Therefore, 80 g of Oxygen will contain = 80/ 16 = 5 moles.
(iii) Gram molar mass of C12H22O11 (Sucrose) is 342 g So, 342 g of sucrose contains 1 mole of it Therefore, 10 g will contain 10/342 = 0.029 mole
![]()
Question 31.
Write the formula of the following compounds and calculate the molecular mass:
(i) Ammonium Oxalate
(ii) Sodium Sulphate
(iii) Aluminium Nitrate
Answer:
(i) Ammonium Oxalate-(NH4)2C2O4Molecular mass = (2 x (1 x atomic mass of N + 4 x atomic mass of H) + (2 x atomic mass of C) + (4 × atomic mass of O)
= 2(14+4) +(2 × 12) +(4 × 16)
= 36 + 24+ 64 = 124 g/mol
(ii) Sodium Sulphate :Na2SO4
Molecular mass = (2 x atomic mass of Na + 1 x atomic mass of S + 4 x atomic mass of O)
= (2 × 23 + 1 × 32 + 4 × 16)
= (46 + 32 + 64) = 142 g/mol
(iii) Aluminium Nitrate : Al(N03)3
Molecular mass = (1 x atomic mass of A1 + 3 (1 × atomic mass of N + 3 × atomic mass of O)
= 26.9+3(14+3 × 16)
= 26.9+ 3 × 62
= 26.9+ 186
= 212.9 g/mol
Question 32.
What is the number of atoms present in 32 g of oxygen gas and 14 g of nitrogen gas?
Answer:
Gram atomic mass of oxygen is 16 g
So, 16 g oxygen contains 6.023 × 1023 atoms
Hence 32 g oxygen will contain 6.023 × 1023 × 2 atoms
=12.046 x 1023
Now, gram atomic mass of Nitrogen = 14 g
So, 14 g nitrogen contains 6.023 × 1023 atoms
![]()
Question 33.
Calculate molar mass of the following:
(i) HNO3
(ii) CO2
(iii) C2H6
Answer:
(i) Molar mass of HNO3 = (1 × atomic mass of H +1 × atomic mass of N + 3 atomic mass of O)
= (1 × 1) + (14 x 1) + (16 × 3)
= 1 + 14 + 48= 63 g/mol
(ii) Molar mass of CO2 = (1 x atomic mass of C + 2 × atomic mass of O)
= 1(1 × 12)+ (2 × 16)
= 12 + 32= 44 g/mol.
(iii) Molar mass of C2H6 = (2 x atomic mass of C + 6 × atomic mass of H) = ( 2 × 12) + (6 × 1) = 24 + 6
= 30 g/mol
Question 34.
Which of the following contains maximum number of molecules :
(i) 36 g water
(ii) 28 g carbon monoxide
Answer:
(i) Molar mass of water (H2O) is 18 g/mol 18 g of water contains 1 mole which contains 6.023 × 1023 molecules of it.
Therefore, 36 g water will contain 2 moles i.e, 6.023 × 1023 × 2 molecules
= 12.046 × 1023molecules
(ii) Molar mass of carbon monoxide (CO) is 28 g/mol So, 28 g of CO will contain 1 mole i.e, 6.023 × 1023 molecules
Therefore 36 g of water will contain maximum number of molecules.
![]()
Question 35.
Which of the following contains minimum number of molecules?
(i) 46 g of ethyl alcohol
(ii) 54 g of nitrogen pentaoxide
Answer:
(i) Molar mass of ethyl alcohol (C2H5OH) is 46 g/mol
So, 46 g of ethyl alcohol will have 6.023 × 1023 molecules,
(ii) Molar mass of nitrogen pentaoxide (N2O5) is 108 g/mol
So, 54 g of N2O5 will contain 1/ 2 mole
i. e., 6.023 × 1023 × (1/2) molecules.
= 3.0115 × 1023 molecules
Hence, 54 g of nitrogen pentaoxide will have minimum number of molecules.
RBSE Class 11 Chemistry Chapter 1 Long Answer Type Questions
Question 36.
Calculate the mass percentage of different elements present in sodium sulphate.
Answer:
Molecular mass of sodium sulphate (Na2SO4) is 142 g mol.
The elements present in sodium sulphate are sodium, sulphur and oxygen.
Mass of Sodium : 23 × 2 = 46 g
Mass of Sulphur : 32 × 1 = 32 g
Mass of Oxygen : 16 × 4 = 64 g
Mass percentage of sodium = \(\frac{32}{142}\) × 100
= 32.38 or 32.4%
Mass percentage of sulphur = \(\frac{32}{142}\) × 100
= 22.57 or 22.6
Mass percentage of oxygen = \(\frac{64}{142}\) × 100 .
= 45.05%
![]()
Question 37.
What is Limiting reagent? Identify limiting reagent in reaction between 3.0 g H2 and 29 g O2.
Answer:
A limiting reagent is a reactant which is present in lesser amount in a reaction. When it gets consumed, the reaction will not proceed irrespective of the amount of other reactant present in the reaction. It limits the amount of product formed. In other words, it determines the extent of reaction. Consider the reaction between hydrogen and oxygen to form water. It can be represented as:
2H2 + O2 = 2H2O
From the above reaction, it is clear that 2 moles of hydrogen react with 1 mole of oxygen.
Molar mass of H2 = 2 g
Molar mass of O2= 32 g
This means that
4 g of H2 reacts with 32 g of O2
3 g of H2 reacts with = (32/4) x 3 g of O2 gas = 24 g
As the given amount of O2 is more than required.
Therefore, O2 is in excess and H2 is limiting reagent
here.
![]()
Question 38.
Two compounds are formed by carbon and oxygen In one of these, carbon content is 42.9% and in other 27.3%. Verify law of multiple proportion.
Answer:
Step 1: Calculate the percentage composition of carbon and oxygen in each of the two oxides :

Step 2: Calculate the weights of carbon which combine with a fixed weight i.e., one part by weight of oxygen in each of the two oxides. In the first oxide, 57.1 parts by weight of oxygen combine with carbon = 42.9 parts.
1 part by weight of oxygen will combine with carbon
= \(\frac{42.9}{57.1}\)
= 0.751
In the second oxide, 72.7 parts by weight of oxygen combine with carbon = 27.3 parts,
.-. 1 part by weight of oxygen will combine with carbon
=\(\frac{27.3}{72.7}\)
=0.376 parts
Step 3: Compare the weights of carbon which combine with the same weight of oxygen in both the oxides— The ratio of the weights of carbon that combine with the same weight of oxygen (1 part) is 0.751 : 0.376 or 2 : 1 Since this is a simple whole.number ratio, so the above data illustrate the law of multiple proportions.
Question 39.
Write short notes on:
(i) Dalton’s Atomic Theory
(ii) Gay Lussac’s Law of Combining Volumes
(iii) Avogadro’s Hypothesis and its applications.
Answer:
(i) Dalton’s Atomic Theory: Main postulates of
Dalton’s atomic theory are as follow :
- Matter is composed of very tiny or microscopic particles called “Atom”.
- Atom is an indivisible particle.
- Atom can neither be created nor be destroyed.
- Atoms of an element are identical in size, shape, mass and in other properties.
- Atoms of different elements are different in their properties.
- Atoms combine with each other in small whole numbers.
- All chemical reactions are due to combination or separation of atoms.
(ii) Gay Lussac’s Law of Combining Volumes: This law states that the volume of gases taking part in a chemical reaction show simple whole number ratio to. one another when those volumes are measured at the same temperature and pressure.
For the reactions :
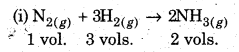
1 volume of nitrogen combines with 3 volumes of hydrogen to form 2 volumes of ammonia.
![]()
2 volumes of hydrogen combine with 1 volume of oxygen to form 2 volumes of steam.
(iii) Avogadro’s-Hypothesis and its applications : In 1811, Italian physicist and mathematician Anedeo Avogadro published a hypothesis (also termed Avogadro’s law or principle) states that the volume of a gas is directly proportional to the number of molecules of the gas. This is represented by the formula ,
V = aN,
where a is a constant, V is the volume of the gas, and N is the number of gas molecules.
Applications
Avogadro’s law has been useful in substantiating a number of important laws and concepts. It has helped in the following areas:
- In explaining Gay Lussac’s law of gaseous volumes.
- In determining the atomicity of gases.
- In determining the molecular formula of a gas.
- In establishing the relationship between relative molecular mass and vapour density.
![]()
Question 40.
Briefly explain Law of chemical combinations.
Answer:
There are five laws of chemical Combinations :
1. Law of Conservation of Mass: This law states that matter can neither be created nor be destroyed. In other words, the total mass, that is, the sum of mass of all reactants and the products formed remains constant. This law is also called as the law of indestructibility of matter. Example
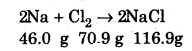
2. Law of Definite Proportions: Joseph Proust, a French chemist stated that the proportion of elements by weight in a given compound will always remain exactly the same. In simple terms, we can say that, irrespective of its source, origin or its quantity, the percent composition of elements by weight in a given compound will always remain the same.
Example: A pure water obtained from which ever source or any country will always be made up of only hydrogen and oxygen elements combined together in the same fixed ratio of 1 : 8 by mass.
3. Law of Multiple Proportions: This law states that if two elements combine to form more than one compound, the masses of these elements in the reaction are in the ratio of small whole numbers. This law was given by Dalton in the year 1803.
For example, Hydrogen and Oxygen combine to form water H20 and hydrogen peroxide H202. Two atoms of hydrogen combine with one atom of oxygen in the case of water, while-two atoms of hydrogen combine with two atoms of oxygen in the case of hydrogen peroxide. The ratio of oxygen atoms combining with a fixed number of hydrogen atoms in these two compounds is 1 : 2.
4. Law of Reciprocal Proportion: This law states that “when two elements combine separately with third element and form different types of molecules, their combining ratio is directly reciprocal if they combine directly.”
5. Gay Lussac’s Law of Gaseous’Volumes: In 1808, Gay Lussac gave this law which states that when gases are produced or combined in a chemical reaction, they do so in simple ratio by volume given that all the gases are at same temperature and pressure.
Example: Combination between nitrogen and hydrogen: One volume of nitrogen always combines With 3 volumes of hydrogen to form two volumes of ammonia. This reaction also indicates a simple ratio of 1: 3 : 2 between the volume of the reactants and products.
RBSE Class 12 Chemistry Chapter 1 Numerical Problems
Question 41.
In an organic compound, 40% carbon, 6.66% hydrogen and rest oxygen is present. Its vapour destiny is 30. Calculate the empirical formula and molecular formula.
Answer:
Percentage of oxygen=100-(40+6.66)=100-46.6=53.4%
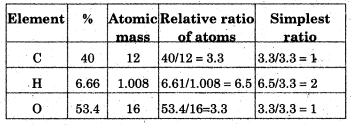
Hence, empirical formula=CH2O
Now, empirical formula mass
= 12 + (2 × 1) + 16 = 12 + 2 +16 = 30″
Molecular mass = 2 x Vapour density = 2 × 30 = 60
n = Molecular mass / Empirical formula mass
= \(\frac{60}{30}\) = 2
Thus, Molecular formula = (Empirical formula) × n = (CH2O) × 2 = C2H4O2
![]()
Question 42.
In an organic compound, the ratio of masses of C, H and N are 9:1:3.5 and molecular mass is 108. What will be the empirical formula and molecular formula of the compound.
Answer:
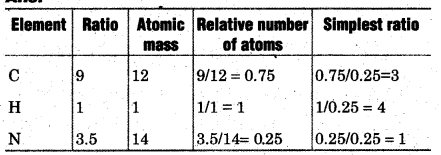
Empirical formula =C3H4N
Empirical formula mass = (3 × 12) + (4 × 1)+ 14 = 54
Thus, Molecular formula of the compound = (Empirical formula) x n
= (C3H4N)2=C6H8N2
Question 43.
In chemical analysis, it is found that in 10 g iron chloride, 3.438 g iron and 6.650 g chlorine are present. Calculate the empirical formula of iron chloride. (Fe = 55.8, Cl= 35.5)
Answer:
Percentage of iron = \(\frac{3.438}{10}\) × 100 = 34.38%
Percentage of Chlorine = \(\frac{6.65}{10}\) × 100 = 66.5%

Thus, the empirical formula of iron chloride is FeCl3
Question 44.
Calculate number of oxygen atoms in 88 g of CO2. Also calculate mass of CO having same number of oxygen atoms.
Answer:
Number of moles of CO2 in 88 g of CO2
= Mass of CO2 / Molar mass of CO2
= 88 g / 44 g/mol = 2 mol CO2
Since one mole of CO2 contains two moles of oxygen atoms, two moles of CO2contain four moles of oxygen atoms. Hence,
1 mole of oxygen atoms contains 6.023 ×1023 oxygen atoms.
4 moles of oxygen atoms contain 6.023 × 1023 × 4 atoms = 2.4092 × 1024 oxygen atoms.
Since 1 mole of oxygen atom is present in 1 mole of CO, 4 moles of oxygen atoms are present in 4 moles of CO.
Mass of 4 moles of CO = number of moles of CO x gram molecular mass of CO
= 4 × (12+ 16)
= 4 × 28
= 112g
![]()
Question 45.
Define atomic mass, molecular mass and equivalent weight. In 500 mL solution, 20.7 g potassium carbonate is dissolved. Calculate its molarity. (Molecular mass of K2CO3=138)
Answer:
Atomic mass: The atomic mass of an element is defined as the number of times an atom of that element is heavier than an atom of carbon-12. It is expressed in a.m.u. or atomic mass unit. In place of a.m.u., symbol u is used which is known as unified mass.
Atomic mass = Mass of an atom × 1 /12 mass of carbon atom
Molecular mass: Molecular mass of any substance is defined as the average relative mass of its molecules as compared to mass of an atom of carbon-12. Molecular mass is expressed as a.m.u. It is given by,
Molecular mass = Mass of one molecule of substance /(1/12) x mass of carbon -12. ,
Equivalent mass: Quantity of a substance in which is numerically equal to its equivalent mass is called its gram equivalent mass.
Calculation of Molarity:
Molar mass of K2CO3= 138 g/mol
Volume of solution = 500 mL or 0.5 L
Mass of K2CO3= 20.7
Number of moles = 20.7/138 = 0.149
Now,
Molarity = Number of moles/Volume of solution
= \(\frac{0.149}{0.5}\) = 0.299 M
Thus, molarity of solution is 0.299 M.
Question 46.
In industrial preparation of nitric acid, how. many moles of N02 are required to form 7.33 moles of HNO3 if the reaction is.
3NO2(g) + H2O(l) → 2HNO3 (l) + NO(g)
Answer:
According to the above equation, 3 moles of NO2 react with one moles form 2 moles of HNO3.
Therefore, number of moles of NO2 produced by 7.33 moles of HNO3, is given as :
3 moles of NO2-+ 2 moles of HNO3,
x moles of NO2-+ 7.33 moles of HNO3
By Cross multiplication, x = 7.33 × 3/2 = 10.99 moles.
![]()
Question 47.
1.68 g iron contains how many moles of iron? Calculate, the number of atoms in same amount of iron. (At. mass of Fe = 56).
Answer:
Number of moles = mass of substance/mass of one mole
= \(\frac{1.68}{56}\) = 0.03 moles
1 mole of iron contains 6.023 × 1023 atoms
0.03 moles of iron will contain 0.03 x 6.023 × 1023 atoms
= 1.8 ×1022 atoms
Question 48.
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + CO2 (g) + H2O (l). What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl ? .
Answer:
1000 mL of.0.75 M HCl have 0.75 mol of HCl = 0.75 x 36.5 g = 24.375 g
(Molar mass of HCl is 36.5)
Mass of HCl in 25 mL of 0.75 M
HCl = 24.375/1000 x 25 g = 0.6844 g
According to the given chemical equation,
CaCO3 (s) + 2HCl(ag) →CaCl2 (aq) + CO2(g) + H2O(Z)
2 mol of HCl i.e. 73 g HCl react completely with 1 mol of CaCO3g i.e. 100 g
.’. 0.6844 g HCl reacts completely with CaCO3 = 100/73 x 0.6844 g = 0.938 g
Question 49.
In laboratory preparation of chlorine, manganese dioxide (Mn02) reacts with aqueous hydrochloric acid according to the reaction:
4HCl (aq) + MnO2 (s) → 2H2O(l) + MnCl2 (aq) + Cl2 (g)
How many grams of HCl react with 5.0 g of manganese dioxide?
Answer:
1 mol of MnO2 = (Atomic mass of Mn + 2 x atomic mass of oxygen)
= 55 + 32 g = 87 g
87g of MnO2 react with 4 moles of HCl i.e. 4 x 36.5 g
= 146 g of HCl.
(Molar mass of HCl is 36.5)
5.0 g of MnO2 will react with HCl = 146/87 x 5.0 g
=8.40 g.
![]()
Question 50.
Hydrochloric acid is 38% by mass. If the density of solution is 1.19 gm cm”3, then what will be the molarity of solution?
Answer:
38% by mass = 38 g HCl in 100 g solution
38 g HCl in 100/1.19 mL = 84 mL solution = 0.084 L .
Molarity = \(\frac{38}{36.5}\) × \(\frac{1}{0.084}\) = 12.39 M.
Thus, the molarity of solution is 12.39 M.