Rajasthan Board RBSE Class 11 Chemistry Chapter 11 p-Block Elements
RBSE Class 11 Chemistry Chapter 11 Text Book Questions
RBSE Class 11 Chemistry Chapter 11 Multiple Choice Questions
Question 1.
Which of the following is a Lewis acid?
(a) PCl3
(b) AlClg
(c) NCl3
(d) ASCl3
Answer:
(b) AlClg
![]()
Question 2.
In which of the following elements +3 oxidation state is most stable?
(a) In
(b) Ga
(c) Al
(d) Tl
Answer:
(c) Al
Question 3.
The nature of aqueous solution of borax is :
(a) Acidic
(b) Neutral
(c) Amphoteric
(d) Basic
Answer:
(d) Basic
![]()
Question 4.
Which of the following does not give Borax Bead test?
(a) Manganese salts
(b) Barium salts
(c) Nickel salts
(d) Cobalt salts
Answer:
(b) Barium salts
Question 5.
What is the formula of dry ice from the following:
(a) CO2
(b) CO
(c) SiO2
(d) Al2O3
Answer:
(a) CO2
![]()
Question 6.
Which of the following is used as a semi conductor?
(a) Carbon
(b) Silicon
(c) Lead
(d) Tin
Answer:
(b) Silicon
RBSE Class 11 Chemistry Chapter 11 Very Short Answer Type Questions
Question 7.
Write the general electronic configuration of p-block elements.
Answer:
The general electronic configuration of p-block elements is ns2 np1-6 .
Question 8.
Which compound of boron is used to make bullet proof jackets?
Answer:
Boron nitride (BN) is used to make bullet proof jacket.
![]()
Question 9.
Write the names and formula of two ores of aluminium.
Answer:
Bauxite : Al2P3 . 2H3O
Cryolite : Na3AlF6
Question 10.
Draw the tetrahedral dimer structure of AlCl3?
Answer:
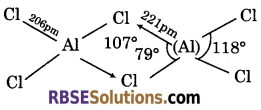
Question 11.
Explain the structure of diborane.
Answer:
Diborane (B2H6) has a three centre electron pair bond. Two types of hydrogen i. e., terminal hydrogen and bridging hydrogen are present in this structure.
Boron—Terminal hydrogen ⇒ Two centre electron pair bond
Boron—Bridging hydrogen ⇒ Three centre electron pair bond.
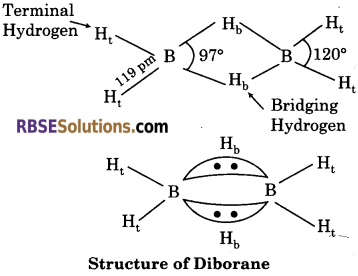
![]()
Question 12.
Write two uses of borane and aluminium.
Answer:
Uses of Borane
- It is used as a reducing agent in organic reactions.
- It is used as a fuel for supersonic rockets.
Uses of Aluminium
- It is used in packing of materials.
- It is used in making utensils.
Question 13.
Write the number of six membered and five membered rings in Fullerenes.
Answer:
In Fullerenes, the number of six membered rings is 20 and five membered rings is 12.
Question 14.
Which allotrope of carbon is thermodynamically most stable?
Answer:
Graphite is thermodynamically most stable allotrope of carbon.
![]()
Question 15.
Explain the hybridisation of carbon present in diamond and graphite.
Answer:
Hybridisation of Carbon in Diamond—In diamond, each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C—C bond length is 154 pm.

Hybridisation of Carbon in Graphite—In grahite, each layer is composed of planar hexagonal rings of carbon atoms. Each carbon atom in hexagonal ring undergoes sp2 hybridisation and makes three sigma bonds with three neighbouring carbon atoms. Fourth electron forms a π-bond. C—C bond length is 14.15 pm.
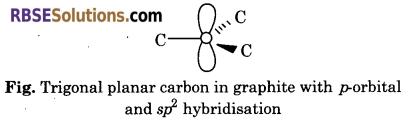
Question 16.
The atomic radius of Ga is less than atomic radius of Al. Why?
Answer:
The atomic radius of Ga is less than atomic radius of Al, because in between Ga and Al, there are ten elements of first transition series which have electrons in inner d-orbital. The inner d-orbital do not shield the nucleus effectively due to their shape and poor penetration power. As a result, effective nuclear charge in Ga becomes more than in Al and atomic radius of Ga is less than Al.
![]()
Question 17.
Why the melting point of Boron is unexpectedly high?
Answer:
Boron has closely packed metallic structure. It also has small size. So, the melting point of Boron is unexpectedly high.
Question 18.
Write the stability of +1 oxidation state of group 13 elements in increasing order.
Answer:
The increasing order of stability of +1 oxidation state of group 13 elements is given below : .
Al < Ga < In < Tl (due to inert pair effect).
Question 19.
Write the reaction for amphoteric nature of aluminium.
Answer:
Aluminium dissolves in mineral acids and aqueous alkalies and so it shows amphoteric nature.
(i) Aluminium dissolves in dilute acid and liberates dihydrogen, which shows its basic nature.
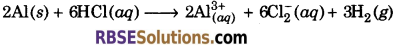
(ii) Aluminium reacts with aqueous alkali and liberates dihydrogen, which shows its acidic nature.
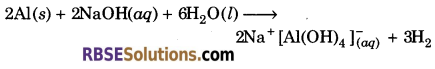
![]()
Question 20.
Why boron can form \(\mathbf{B F}_{4}^{-}\) but not \(\mathbf{B F}_{6}^{-}\)?
Answer:
Boron can form \(\mathbf{B F}_{4}^{-}\) but not \(\mathbf{B F}_{6}^{-}\) because boron does not have d-orbitals so its maximum covalency is four.
Question 21.
Boric acid is considered as a weak acid. Why?
Answer:
Boric acid is considered as a weak acid because it is not able to release H+ ions on its own. It receives OH– ions from water molecule to complete its octet and in turn releases H+ ions.

RBSE Class 11 Chemistry Chapter 11 Short Answer Type Questions
Question 22.
Explain the various oxidation states found in group 14 elements.
Answer:
The group 14 elements have four electrons in outermost shell and its electronic configuration is ns2 np2. They can form M4+ or M4- ions to show ionic character or exhibit tetravalent covalent nature by sharing of four electrons in order to get stability. In the formation of M4+ or M4- ions require huge amount of energy which is normally not possible. So, these elements do not form ionic bond rather they form covalent bonds in both oxidation states. Lead compounds in +2 state are stable and in +4 state are strong oxidising agents.
![]()
Question 23.
PbI2 is formed but not PbI4. Why?
Answer:
PbI2 is formed but not PbI4 because the iodide ion reduces Pb (IV) to Pb (II) and itself gets oxidised to I2 and therefore PbI1 is formed. (I– ion cannot unpair p subshell of Pb)
Question 24.
Explain hydrolysis of SiCl4 .
Answer:
Silicon tetrachloride (SiCl4) undergoes hydrolytic decompostion by water to form silica and hydrochloric acid
SiCl4 + 2H2O ➝ SiO2 + 4HCl
Question 25.
Diamond is hard but graphite is soft and slippery. Explain why?
Answer:
Diamond is hard because the carbon atoms in diamond are bonded in a stronger tetrahedron pattern but graphite is soft and slippery because the carbon atoms in graphite are bonded in layers with only weak vanderwall force holding the layers together. These layers are held together by weak Vander Waals forces, which make graphite soft and slippery.
![]()
Question 26.
CO2 is a gas whereas SiO2 is a solid. Explain with reason.
Answer:
At room temperature, CO2 has only weak dispersion intermolecular forces holding the molecules together. So, CO2 is a gas at room temperature. Whereas in SiO2, each Si-atom is linked with O-atom with single bond thus forming a network solid with high melting point. So, SiO2 is a solid.
Question 27.
Explain the poisonous nature of carbon monoxide.
Answer:
Carbon monoxide (CO) is highly poisonous in nature. Air containing even less than 1% of carbon monoxide can be fatal, if breath it for about 10 to 15 minutes. It combines with Haemoglobin in the blood and form carboxy haemoglobin, which reduces the oxygen carrying capacity of blood. The oxy-haemoglobin carries oxygen to various body parts but when carbon monoxide is combined, it stops the normal circulation of oxygen and may cause death due to suffocation.
Question 28.
Hydrolysis of SiCl4 can take place but not of CCl4 . Explain with reason.
Answer:
In SiCl4 , vacant 3d orbitals are present so it on hydrolysis to silica and hydrochloric acid but in case of CCl4 , there is lack of vacant d -orbitals in C-atom which cannot accept lone pair of electrons from water. So, it cannot undergo hydrolysis.
![]()
Question 29.
Write the formula of unit of Silicones.
Answer:
Silicones are a group of organosilicon polymers which have (R2SiO) as a repeating unit.
Question 30.
In which of the metal the property of catenation is maximum?
Answer:
Maximum tendency of catenation is found in carbon because carbon is small in size and due to this, bond between C—C is strong and p—p overlapping is also strong.
Question 31.
Explain the oxidation state of carbon in Be2 C and Al4 C3.
Answer:
Oxidation state of carbon in Be2 C
Let oxidation state of carbon = x
∴ 2(+2) + x = 0
4 + x= 0
x = – 4
∴ The oxidation state of carbon in Be2C = – 4
Oxidation state of carbon in Al4 C3
Let oxidation state of carbon = x
∴ 4(+3) + 3x = 0
12 + 3x = 0
3x = -12
∴ The oxidation state of carbon in Al4C3 = – 4
![]()
Question 32.
Tl shows (+1) and (+3) oxidation states. Why?
Answer:
Thallium (Tl) shows +1 and +3 oxidation states because on moving down the group 13, the +1 state becomes more stable as compared to +3 due to inert pair effect. The two electrons present in the s-shell (ns2np1) are strongly attracted by the nucleus and do not participate in bonding.
Question 33.
Why Boron forms electron deficient compounds?
Answer:
Boron forms electron deficient compounds because of small size and high charge density of boron. Boron has very small atomic radius. Boron forms covalent compounds. It has 3 valence electrons which it prefers to share rather than donate as B3+ ions is very small and unstable, so it forms sextet rather than octet. Due to incomplete octet of boron, it forms electron deficient compounds.
Question 34.
The halides of boron do not form dimer but AlCl3 form dimer (Al2Cl6 ). Why?
Answer:
BCl3 and AlCl3 both are the electron deficient compounds, as there are only six electrons in the valency shell of central atom after the formation of the molecule. So,both the compounds have strong tendency to gain two electrons, so that their octet is complete.
The electron deficiency of boron in BCl3 is compensated by formation of co-ordinate bond between the lone pair of peripheral chlorine atom and the empty unhybridised p-orbital of Boron atom, forming pn – pn bonding (Back bonding). So, the electron deficiency of BCl3 is compensated by formation of pn – pn bonds within the molecules hence halides of boron i. e., BCl3 do not form dimer.
However the electron deficiency of Al-atom in AlCl3 is compensated by formation of co-ordinate bond between lone pair of chlorine atom of another AlCl3 molecule and the empty unhybridised p orbital of Al-atom. So, AlCl3 forms dimer as Al2Cl6.
![]()
Question 35.
Why property of catenation decreases down the group in group 14?
Answer:
The property of catenation decreases down the group in group 14 due to increase in atomic size and decrease in electronegativity. The order of catenation is given as below :
C >> Si > Ge ≃
Lead does not show catenation.
Question 36.
What are silicones? How are they prepared?
Answer:
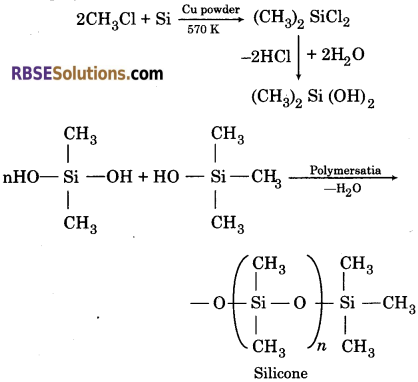
Silicones-They are a group of organosilicon polymers, which have (R2SiO) as a repeating unit. Preparation of Silicones-The starting materials for the manufacture of silicones are alkyl or aryl substituted silicon chlorides RnSiCl4-n, where R is alkyl or aryl group.
When methyl chloride reacts with silicon in the presence of copper as a catalyst at a temperature 570 K, various types of methyl substituted chlorosilanes are formed. Hydrolysis of dimethyl dichloro silane i. e., (CH3)2 SiCl2 followed by condensation polymerisation forms straight chain polymer i. e., silicone.
Question 37.
Write the formula of water gas and producer gas.
Answer:
Water gas : CO + H2
Producer gas : CO + N2
![]()
Question 38.
What happens when formic acid is heated with concentrated sulphuric acid. Give equation also.
Answer:
When formic acid is heated with concentrated sulphuric acid, it forms carbon monoxide and water.

RBSE Class 11 Chemistry Chapter 11 Long Answer Type Questions
Question 39.
Write short notes on :
(i) Zeolites
(ii) Silicates
(iii) Silicones.
Answer:
(i) Zeolites—zeolites are microporous crystalline solids with well-defined structures. Generally, they contain silicon, aluminium and oxygen in their framework and cations, water and/or other molecules within their pores.
Zeolite is the broad term used to describe a family of minerals called tectosilicates. These minerals contain small pores which provide a general surface area. These molecule contain tetrahedral \(\mathrm{AlO}_{4}^{5-}\) and \(\mathrm{Si} \mathrm{O}_{4}^{4-}\) molecules bound by oxygen atoms.
Zeolites are widely used as a catalyst in petro-chemical industries for cracking of hydrocarbons and isomerization, e.g., ZSM-5 used to convert alcohols directly in to Gasoline. Hydrated zeolites are used as ion exchangers in the softening of hard water.
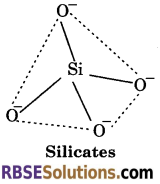
(ii) Silicates—The basic structural unit of silicates is Si04 in which silicon atom is bonded to four oxygen atoms in tetrahedron fashion. In silicates, either the discrete unit is present or a number of such units are joined via corners by sharing 1, 2, 3 or 4 oxygen atoms per silicate units. When silicate units are linked together, they form chain, ring, sheet or three dimensional structures. Negative charges on silicate structure is neutralised by positively charged metal ions. If all the four corners are shared with other tetrahedral units, it forms three-dimensional network. e.g., Feldspar, Zeolites, Mica and Asbestos. Man-made silicates are glass and cement.
(iii) Silicones—Silicones are a group of organosilicon polymers, which have (R2SiO) as a repeating unit.
Silicones being surrounded by non-polar alkyl groups are water repelling in nature. They have high thermal stability, high dielectric strength and resistance to oxidation and chemicals. The starting materials for the manufacture of silicones are alkyl or aryl substituted silicon chlorides, RnSiCl(4-n) where R is alkyl or aryl group.
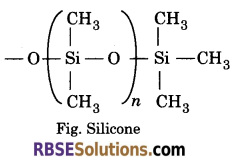
Silicones are used as greases, electrical insulators and for water proofing of fabrics. Being biocompatible, they are also used in surgical and cosmetic plants.
![]()
Question 40.
Explain the following:
(i) Catenation
(ii) Inert Pair Effect
(iii) Allotropy.
Answer:
(i) Catenation—It is the linkage of atoms of the same element into longer chains. Catenation occurs most readily in carbon, which forms covalent bonds with other carbon atoms to form longer chains and structures. This property is due to strong C—C bonds.
(ii) Inert Pair Effect—The reluctance of s electrons in participating in chemical bonding. It is due to the poor shillding of d and f block electrons of inner shell which increases the effective nuclear charge.
The term ‘inert pair’ was first proposed by N. Sidgwick in 1927. The name suggests that the s electrons are more tightly bound to the nucleus and therefore more difficult to ionize.
For example, the p-block elements of the 4th, 5th and 6th period come after d-block elements but the electrons present in the intervening d- (and f-) orbitals do not effectively shield the s-electrons of the valence shell. Due to this, the inert pair of ns electrons remains more tightly held by the nucleus and therefore participates less in bonding.
(iii) Allotropy—Allotropy is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of these elements. Allotropes are different structural modifications of an element, the atoms of the elements are bonded together in a different manner.
For example, the allotropes of carbon include diamond, graphite and fullerenes.
![]()
Question 41.
Write the balanced chemical equation :
(i) ZnO + CO ➝
(ii) C + H2O ➝
(iii) B2H6 + O2 ➝
(iv) H3BO3 ➝
(v) Al + NaOH ➝
(vi) BF3 + NH3 ➝
Answer:
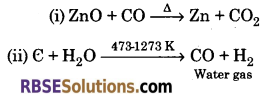
(iii) 2H3BO3 ➝ B2O3 + 3H2O

(v) 2Al + 2NaOH + 6H2O ➝ 2Na[Al(OH)4] + 3H2
(vi) BF3 +NH3 ➝ [B(NH3)F3]
Question 42.
What are electron deficient compounds? Are BCl3 and SiCl4 electron deficient compounds? Explain.
Answer:
Electron deficient compounds are those compounds in which there are only six electrons in the valency shell of central atom after the formation of the molecule. Electron deficient bonds are often described as 3-center-2-electron bonds.
BCl3 is electron deficient compound because boron has only 6 valence electrons and is short of 2 electrons. Therefore, it can accept a pair of electrons from nucleophiles. SiCl4 is not electron deficient compound because silicon has 8 valence electrons.
![]()
Question 43.
Explain the following with reason :
(i) Lead (II) chloride reacts with Cl2 to give PbCl4.
(ii) Lead (IV) chloride is highly unstable towards heat.
(iii) Concentrated nitric acid can be of transported in aluminium container.
(iv) Graphite is used as a lubricant.
Answer:
(i) Lead (II) chloride reacts with Cl2 to give PbCl4 because lead exists in +2 and +4 oxidation states.
PbCl2 + Cl2 ➝ PbCl4
(ii) Lead tetrachloride (PbCl4) is highly unstable towards heat because the stability of +4 oxidation state decreases on moving down the group in the periodic table due to inert pair effect. So, +2 oxidation state of Pb is more stable. It decomposes on heating to give lead dichloride and chlorine gas.

(iii) Concentrated nitric acid reacts with aluminium to form a very thin layer of aluminium oxide which acts as a protective layer and protects from further reaction of nitric acid with aluminium. Due to this protective layer aluminium cannot react further with cone. HNO3. Hence, concentrated nitric acid can be transported in aluminium container.
(iv) Graphite has layered structure in which different layers are held together by weak Vander Waals forces and so each layer can slip over one another and hence graphite is used as a lubricant.