Rajasthan Board RBSE Class 11 Chemistry Chapter 13 Hydrocarbons
RBSE Class 11 Chemistry Chapter 13 Text Book Questions
RBSE Class 11 Chemistry Chapter 13 Multiple Choice Questions
Question 1.
The number of 3°, 2° and 1° Carbon in isopentane :
(a) 1, 9, 2
(b) 9, 1, 2
(c) 2, 1, 9
(d) 1, 2, 9
Answer:
(d) 1, 2, 9
![]()
Question 2.
The alkane obtained by Wurtz reaction of the product of reaction between 2-butene with HBr is :
(a) Single branched
(b) Double branched
(c) Triple branched
(d) Unbranched
Answer:
(b) Double branched
Question 3.
Which of the following groups do not show addition reactions?
(a) Alkanes
(b) Alkaloids
(c) Cycloalkenes
(d) Ketones
Answer:
(a) Alkanes
![]()
Question 4.
Which of the following will not react with methane under normal conditions?
(a) I2
(b) Cl2
(c) Br2
(d) F2
Answer:
(a) I2
Question 5.
In which of the following reaction of alkene with HX, carbanion will be formed quickly ?
(a) HI
(b) HBr
(c) HCl
(d) All the above
Answer:
(a) HI
RBSE Class 11 Chemistry Chapter 13 Very Short Answer Type Questions
Question 6.
What are paraffins and olefins? Give one example of each and write chemical reaction to differentiate them.
Answer:
Paraffins : Paraffins is a Latin word which means (Parum = little + affinis = reactivity). These are saturated hydrocarbons, which are also called alkanes. They contain carbon-carbon single bonds. There general formula is CnH2n + 2.
Example : Methane (CH 4)
Olefins : Olefins means oil forming. These are unsaturated hydrocarbons containing atleast one double bond. These are also called alkenes. Their general formula is Cn H 2n.
Example : Ethylene (C2H 4)
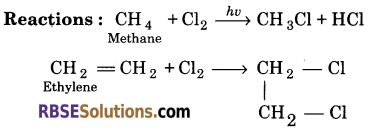
![]()
Question 7.
Write general formula of alkenes and explain general method for its preparation.
Answer:
The general formula of alkenes is CnH2n.
General method of preparation of alkene :
Alkenes are generally prepared by dehydration of alcohols, These are prepared by passing the vapours of an alcohol over heated alumina at 623 K.
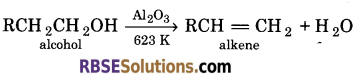
Question 8.
Write the chemical equation for the formation of ethene in one step only by—
(a) Ethanol
(b) Ethyl bromide
(c) Ethyne
(d) Ethylene dibromide
Answer:
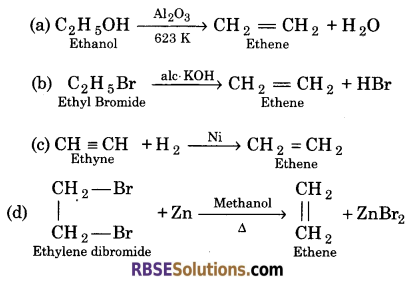
Question 9.
Explain Markownikoff’s rule and give examples also.
Answer:
Markownikoff’s Rule : According to this rule, “the negative part of the unsymmetrical reagent attaches to that carbon atom of the double bond of unsymmetrical alkene which bears the least number of hydrogen atoms.”
Example :

![]()
Question 10.
Explain the addition of HBr to alkenes according to Markownikoffs rule.
Answer:
The negative part i.e., Br of HBr attaches to the carbon atom of the double bond of alkene i,e., propene which bears the least number of hydrogen atoms. This is due to presence of electron releasing group
Example : -CH3 group in propene which shows +I effect and from more stable carbocation i.e.,
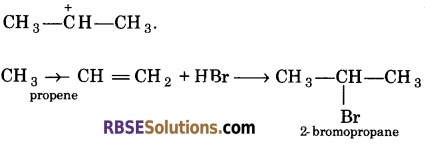
Question 11.
Write the name and formula of first three members of acetylene series.
Answer:
C2H2 Acetylene CH ≡ CH
C3H4 Propylene CH3—C ≡ CH
C4H6 Butylene CH 3—C ≡ C—CH3
Question 12.
Write the general formula of alkene and alkyne and give a chemical test to distinguish them.
Answer:
General formula of alkene = Cn H 2n
General formula of alkyne = Cn H 2n – 2
When ammonical cuprous chloride solution is added to an alkene, there is no effect. Where as when ammonical cuprous chloride solution is added to acetylene, red precipitate of cuprous acetylide is formed.
![]()
Question 13.
What are 3°, 2° and 1° hydrogen? Explain with examples.
Answer:
3°, 2° and 1° hydrogen are the hydrogen which are attached with 3°, 2° and 1° carbon respectively in
a hydrocarbon. Example : Isopentane
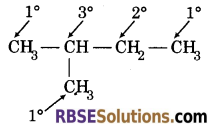
In this compound, 3°, 2° and 1° carbon contain 1, 2 and 9 hydrogen respectively.
Question 14.
Draw tetrahedral structure of methane and give bond angle between H—C—H.
Answer:
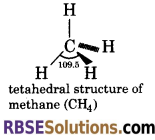
Bond angle between H—C—H in methane is 109.5° or 109°28′.
![]()
Question 15.
Write the chemical equation for formation of acetylene in one step only.
(a) Ethyl dibromide
(b) Trichloromethane
(c) Ethylidene dichloride
(d) Acetylene tetrabromide
Answer:
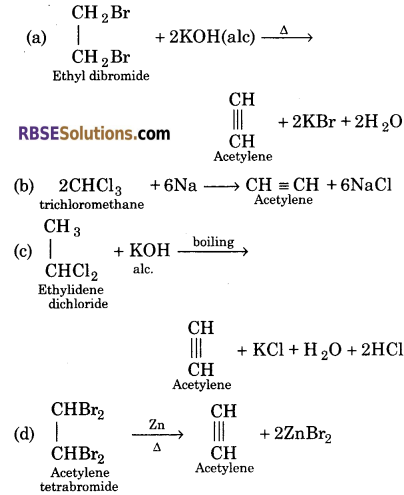
Question 16.
Give two chemical tests to detect unsaturation in organic compounds.
Answer:
- Bromine test: When 5% solution of bromine in carbon tetrachloride is added to an alkene, it gets decolourized. This Indicate the presence of unsaturation in organic compound.
- Bayer’s test: When alkene is oxidised with cold,. alkaline KMnO4, diol is formed. The KMnO4 gets decolourized. This indicates the presence of unsaturation of organic compound.
![]()
Question 17.
Give reaction : What happens when :
(a) Ethyl alcohol is heated with excess of concentrated sulphuric acid at 1600°C ?
(b) Passing ethene in cold aqueous solution of alkaline potassium permanganate.
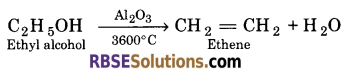
(c) Vapours of ethyl alcohol are passed over hot Aluminium oxide at 3600°C. ?
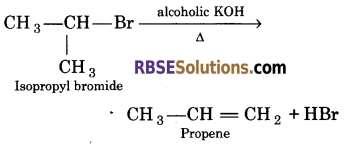
(d) Isopropyl bromide is heated with alcoholic KOH ?
(e) High pressure is applied on ethylene in the presence of peroxide catalyst ?
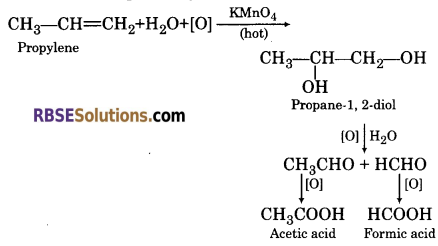
(f) Passing propylene in hot solution of potassium permanganate solution?
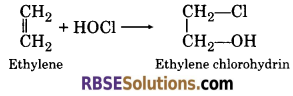
(g) Reaction of ethylene and hypochlorous acid ?
(h) Ozone is treated with ethylene ?
Answer:
(a) When ethyl alcohol is heated with excess of concentrated sulphuric acid at 1600°C, ethene is formed.
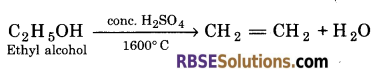
(b) On passing ethene in cold aqueous solution of alkaline potassium permanganate, KMn04 gets decolourized and ethane -1, 2-diol is formed.

(c) When vapours of ethyl alcohol are passed to hot aluminium oxide at 3600°C, ethene is formed.
(d) When isopropyl bromide is heated with alcoholic KOH, propene is formed.
(e) When high pressure is applied on ethylene in the presence of peroxide catalyst, it undergoes polymerization to form polyethene.

(f) On passing propylene on hot solution of potassium permanganate solution, diol is formed which further oxidise to form acetaldehyde and formaldehyde, which on further oxidation forms acetic acid and formic acid respectively.
(g) When ethylene reacts with hypochlorous acid, it forms ethylene chlorohydrin.
(h) When ozone is treated with ethylene, ethylene ozonide is formed.
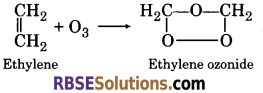
![]()
Question 18.
How following compounds are formed from ethylene :
(1) Acetylene;
(2) Formaldehyde ;
(3) Ethylene glycol;
(4) Ethylene chlorohydrin;
(5) Ethyl alcohol;
(6) Ethylene oxide ;
Answer:
(1) When ethylene undergoes oxidation, it forms acetylene.
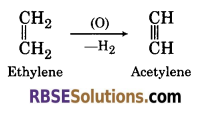
(2) When ethylene reacts with ozone, it forms ethylene ozonide which on hydrolysis with water in the presence of Zn form formaldehyde.
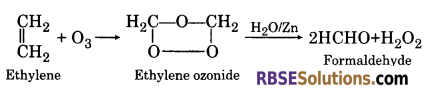
(3) When ethylene is passed in cold aqueous solution of potassium permanganate, it forms, ethylene glycol.

(4) When ethylene reacts with hypochlorous acid, it forms ethylene chlorohydrin

(5) When ethylene reacts with water in the presence of dilute acid at 300°C and 65 atmospheric pressure, it forms ethyl alcohol.

(6) When ethylene is mixed with air and passed under pressure over a silver catalyst at 200-420°C, it forms ethylene oxide.

![]()
Question 19.
Compare ethane, ethene and acetylene carbon-carbon bond on the basis of bond length, stability and reactivity.
Answer:
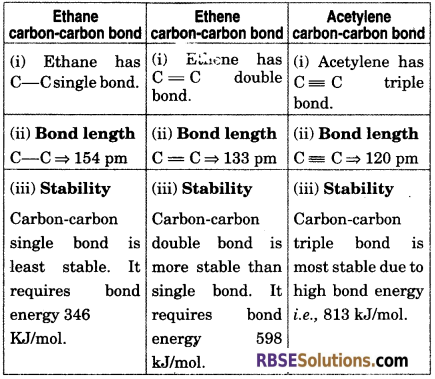
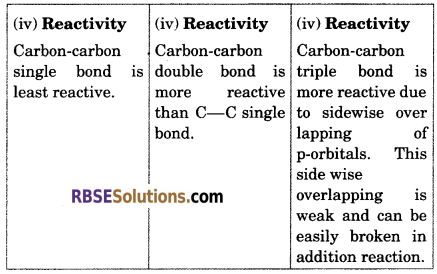
Question 20.
Write short notes on :
(a) Bromination of ethylene
(b) Polymerization of ethylene
(c) Markownikoff s rule
(d) Ozonolysis
Answer:
(a) Bromination of ethylene :
Ethylene reacts with bromine to form 1, 2-dibromo ethane.
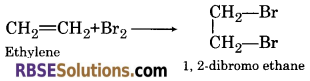
(b) Polymerization of ethylene :
Ethylene undergoes addition polymerization, when heated under pressure, in the presence of peroxide catalyst.

(c) Markownikoffs rule :
According to this rule, “the negative part of the unsymmetrical attacking regent attaches to that carbon atom of the double bond of unsymmetrical alkene which bears the least number of hydrogen atoms.”
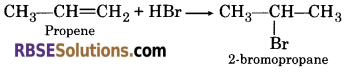
(d) Ozonolysis :
When ethylene reacts with ozone, it gives ethene ozonide
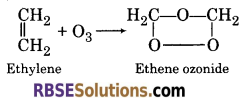
![]()
Question 21.
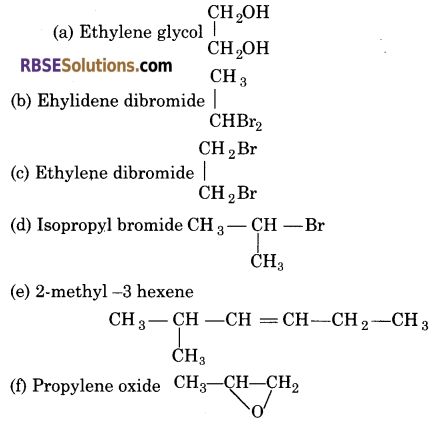
Write the structural formula of the following:
(a) Ethylene glycol
(b) Ethylidene dibromide
(c) Ethylene dibromide
(d) Isopropyl bromide
(e) 2-methyl -3 hexene
(f) Propylene oxide
Answer:
RBSE Class 11 Chemistry Chapter 13 Short Answer Type Questions
Question 22.
What is peroxide effect? Explain giving one example.
Answer:
The addition of HBr to unsymmetrical alkenes against the Markownikoff s rule is called peroxide effect or Anti Markownikoff s rule. According to this effect, “the negative part of the unsymmetrical attacking reagent attached to that carbon atom of the double bond of unsymmetrical alkene which bears the higher number of hydrogen atoms.” This reaction takes place in the presence of peroxide.

![]()
Question 23.
Draw the structure of stereoisomers obtained by reaction of cis-2-butene with bromine.
Answer:
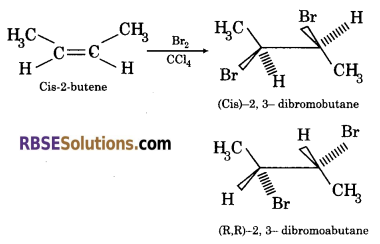
Question 24.
Give the structure and name of the product obtained by the reaction of 1-butene with bromine.
Answer:
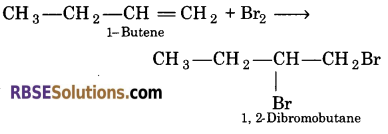
Question 25.
The central carbon-carbon bond in 1, 3-butadiene is shorter than n-butane. Why?
Answer:
The central carbon-carbon bond in 1, 3-butadiene is shorter than n-butane because of the presence of double bonds in 1, 3-butadiene.
![]()
Question 26.
Explain with chemical equation. What happens when :
1. Passing acetylene gas in solution of ammonical cuprous chloride ?
2. Passing acetylene gas in red hot tube ?
3. Acetylene is passed in dilute sulphuric acid ?
4. Acetylene is passed in hydrochloric acid in the presence of mercuric sulphate ?
5. Acetylene is passed in aqueous solution containing Hg++ and H+ ions?
6. Acetylene gas is passed in hypochlorous acid ?
Answer:
(1) On passing acetylene gas in solution of ammonical cuprous chloride, acetylene undergoes linear polymerization.
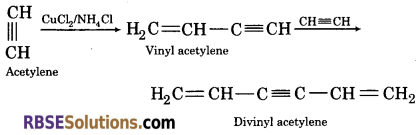
(2) On passing acetylene gas in red hot tube, it polymerizes to benzene.
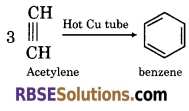
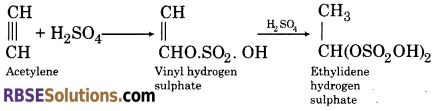
(3) When acetylene is passed in dilute sulphuric acid, it forms ethylidene hydrogen sulphate.
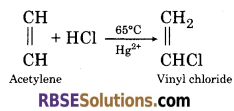
(4) When acetylene is passed on hydrochloric acid in the presence of mercuric sulphate, it gives vinyl chloride.
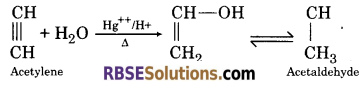
(5) When acetylene is passed in aqueous solution containing Hg++ and H + ions, it gives acetaldehyde.
(6) When acetylene is passed in hypochlorous acid, it gives 1, 1-dichloro acetaldehyde.
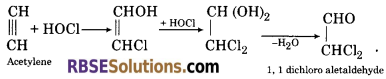
![]()
Question 27.
Distinguish between the following. Give chemical equation also.
1. Ethylene and Acetylene
2. Ethane and Ethyne
3. Saturated and unsaturated hydrocarbons
4. 1-butene and 1-butyne
Answer:
(1) When ammonical silver nitrate solution is added to ethylene, there is no effect. While when ammonical silver nitrate solution is added to acetylene it forms white precipitate of silver acetylide.
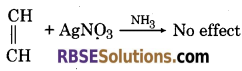
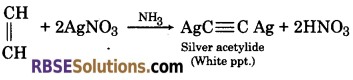
(2) Ethane and ethyne can be distinguished by bromine test. When bromine water is added to ethane, there is no effect. Whereas, when bromine water is added to ethyne, it gets decolourized.
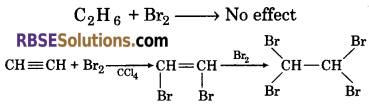
(3) When a 5% solution of bromine in carbon tetrachloride is added to an organic compound, it gets decolourized. This indicates the presence of unsaturation in compound. While saturated hydrocarbon do not give this test.
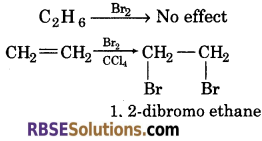
(4) When ammonical silver nitrate is added to an organic compound, 1-butyne will give a white precipitate. While 1-butene does not react.
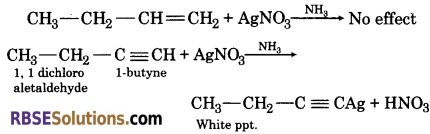
![]()
Question 28.
Explain industrial method for preparation of acetylene. Also give chemical equation also.
Answer:
Acetylene is prepared on large scale by the reaction of water on calcium carbide. Calcium carbide is prepared by heating quicklime with coke.
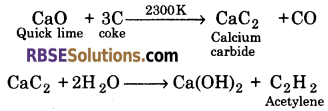
Acetylene can also be prepared on industrial scale by pyrolysis of methane at very high temperature of about 1800 K.

Question 29.
Draw geometry of acetylene molecule and explain the nature of bonds present in the molecule.
Answer:
Geometry of Acetylene : Each carbon atom of acetylene has two sp hybridised orbitals. Carbon-carbon (σ)-bond is obtained by the head-on overlapping of the two sp-hybridized orbitals of the two carbon atoms. The remaining sp-hybridzied orbital of each carbon atom undergoes overlapping along the internuclear axis with Is orbital of each of the two hydrogen atoms forming two C—H sigma bonds. H—C—Cbond angle is 180°.
The acetylene molecule consists of one C—C single bond, two C—H bonds and two C—C double bonds. The strength of C = Cbond is more than C = Cbond, The bond energy of C = C bond is 823 KJ/mole.
The geometry of acetylene is linear. It is shown as below :—
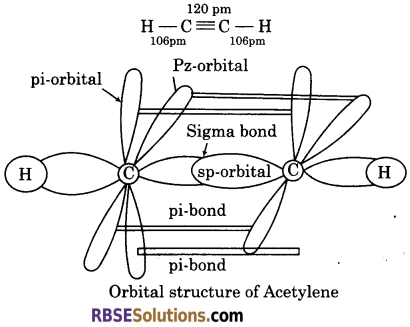
![]()
Question 30.
Explain with chemical equation what happens when:
1. Acetylene reacts with bromine water ?
2. Hydrogen bromide is added to acetylene.
3. Acetylene is passed in cold dilute alkaline potassium permanganate solution ?
4. Hydrolysis of the product obtained by reaction of acetylene and ozone in carbon tetrachloride solution ?
Answer:
(1) When acetylene.reacts with bromine water, it gets decolourized and form dibromo derivative.
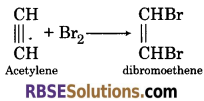
(2)When hydrogen bromide is added to acetylene, it forms ethylidene dibromide.
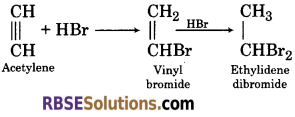
(3) When acetylene is passed in cold dilute alkaline potassium permanganate solution, it gets oxidise to form oxalic acid.
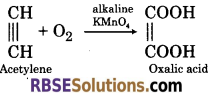
(4) When acetylene reacts with ozone, it forms ozonide which on hydrolysis gives glyoxal and then formic acid.
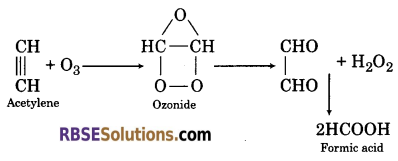
Question 31.
The boiling point of re-pentane is higher than neopentane. Explain the reason.
Answer:
The boiling point of re-pentane is higher than neo pentane because with the increase in number of branched chain, the molecule attains spherical shape. This results in smaller area of contact and so weak intermolecular forces between spherical molecules, which decreases the boiling point of neopentane.
RBSE Class 11 Chemistry Chapter 13 Long Answer Type Questions
Question 32.
Give the structure and IUPAC name of the different alkanes obtained by reaction of sodium with 1-bromo propane and 2-bromo propane in the presence of ether. What is the name of the reaction?
Answer:
When 1-bromo propane reacts with sodium in the presence of ether, n-hexane is formed.
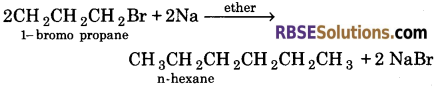
IUPAC name of product ⇒ Hexane
When 2-bromopropane reacts with sodium in the presence of ether, 2, 3-dimethyl butane is obtained.
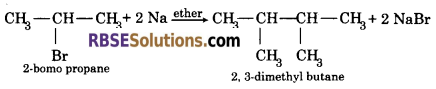
IUPAC name of product ⇒ 2,3-dimethyle butane
![]()
Question 33.
What are alkanes? Write general formula of alkanes and four general methods for preparation of alkanes.
Answer:
Alkanes : Alkanes are saturated open chain hydrocarbons containing carbon-carbon single bonds. These are also known as paraffins.
The general formula of alkanes in Cn H 2n + 2 where n stands for number of carbon atoms and 2n + 2 for number of hydrogen atoms in the molecule.
Example : CH 4 (methane),
C2H6 (ethane) etc.
General methods for preparation of alkanes :
(1) From unsaturated hydrocarbons : The unsaturated hydrocarbons are converted into alkanes by catalytic hydrogenation in the persence of catalyst such as Ni, Pt or Pd at 150°-200° C.
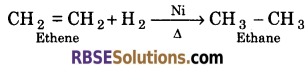
(2) From alkyl halides : When an alkyl halide is treated with sodium in the presence of dry ether, alkane is obtained. This reaction is called wurtz reaction.

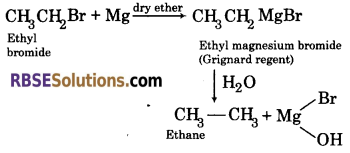
(3) By the use of Grignard reagent: Alkyl halide on treating with magnesium in the presence of dry ether forms alkyl magnesium halide. It is called Grignard reagent. It undergoes hydrolysis to from alkane.
(4) By Cleinmensen reduction : When an aldehyde or ketone is refluxed with 40% aqueous hydrochloric acid, amalgamated zinc and an organic solvent like, toluene, it undergoes reduction to form alkane.
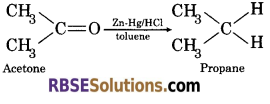
![]()
Question 34.
Write the common name, IUPAC name and formula of isomers of butane, pentane and hexane.
Answer:
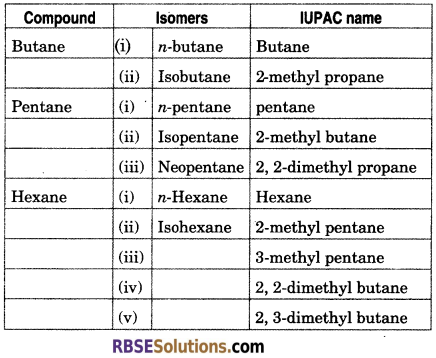
Question 35.
Why alkanes are chemically inert? Explain general reaction of alkanes.
Answer:
Alkanes are chemically inert due to presence of C—H and H—H bond and polar nature. They do not react with acid, base, oxidation, reduction etc. in normal conditions.
General reaction of alkanes :
(i) Combustion : Alkanes burn readily with non-luminous flame in the presence of air or oxygen to form CO2 and water with the evolution of heat.
eg. CH4 + 2O2 ➝ CO2 + 2H 2O, ∆H = -ve
(ii) Halogenation: When alkanes react with halogen such chlorine, the replacement of H-atom of alkane by halogen takes place and it forms alkyl halide.
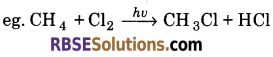
(iii) Isomerization : n-alkanes on heating in the presence of anhydrous aluminium chloride and HCl gas isomerise to branched chain alkanes.
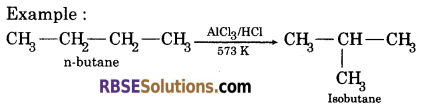
(iv) Pyrolysis : Higher alkanes on heating to higher temperature decompose into lower alkanes, alkene etc.
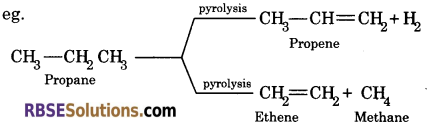
![]()
Question 36.
Explain with chemical equation what happens when ?
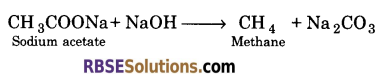
1. Dry sodium acetate is heated with soda lime ?
2. Reaction of sodium with methyl iodine in dry ether solution ?
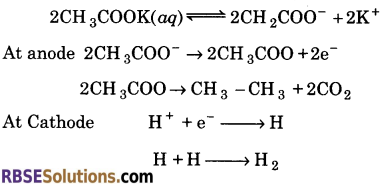
3. Electrolysis of concentrated aqueous solution of potassium acetate ?
4. Aluminium carbide is treated with water ?
Answer:
(a) When dry sodium acetate is heated with sodaline (NaOH + CaO), methane is formed.
(b) When methyl iodide reacts with sodium in ether, ethane is formed.

(c) When concentrated aqueous solution of potassium acetate is electrolyed, ethane is evolved at the anode.
(d) When aluminium carbide is treated with water, it forms aluminum hydroxide and methane.
