Rajasthan Board RBSE Class 11 Chemistry Chapter 8 Oxidation-Reduction Reactions
RBSE Class 11 Chemistry Chapter 8 Text Book Questions
RBSE Class 11 Chemistry Chapter 8 Multiple Choice Questions
Question 1.
Oxidation is a process :
(a) In which electrons are accepted
(b) In which electronegative element is added
(c) In which electro positive element is added
(d) In which oxygen is released
Answer:
(b) In which electronegative element is added
![]()
Question 2.
In which of the following pair of compounds, oxidation number of chromium is same
(a) K2CrO4 and KCrO2
(b) KCrO2 and Cr(CO)6
(c) K2Cr2O7 and Cr(CO)4
(d) K2Cr2O7 and K2CrO4
Answer:
(b) KCrO2 and Cr(CO)6
Question 3.
The oxidation number of carbon in diamond is :
(a) Zero
(b) +4
(c) -4
(d) +2
Answer:
(a) Zero
![]()
Question 4.
In the following reaction 2FeCl3 +SnCl2 ➝ 2FeCl2 +SnCl4
The substance which get reduced is :
(a) Sn+2
(b) Fe+2
(c) Sn+4
(d) Fe+3
Answer:
(d) Fe+3
Question 5.
Which of the following is a redox reaction ?
(a) 2FeCl3 + SnCl2 ➝ 4 2FeCl2 + SnCl4
(b) ASNO3 + HCl ➝ AsCl + HNO3
(c) 2Kl + Pb(NO)3 ➝ 2KNO3 +Pbl2
(d) BaCl2 + H2SO4 ➝ BaSO4 + 2HCl
Answer:
(a) 2FeCl3 + SnCl2 ➝ 4 2FeCl2 + SnCl4
RBSE Class 11 Chemistry Chapter 8 Very Short Answer Type Questions
Question 6.
What is cell potential ?
Answer:
The cell potential is the measure of potential difference between two half cell in an electrochemical cell. It is represent by the symbol E-cell.
Question 7.
Which is of two F or I shows both positive and negative oxidation states ?
Answer:
I (iodine) shows both positive and negative oxidation states.
Question 8.
Calculate the value of x in the following equation :
CIO– + H2O + xe– ➝ + Cl + 20H–
Answer:
The value of x is 2 in the given reaction :
CIO– +H2O + 2e– ➝ Cl– + 20H–
gain of two electrons in this reaction :
![]()
Question 9.
Identify the reducing agent in the following reaction:
5H2O2 +Br2 ➝ 2HBrO3 +4H20
Answer:
Br2 acts as reducing agent in the given reaction, because it reduces hydrogen peroxide into water.
H2O2 + 2H+ + 2e– ➝ 2H2O (reduction)
Question 10.
In the given structure the electronegativity of Y is more then Z. Find the oxidation Number of Z.
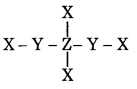
Answer:
In this structure, the oxidation number of Z is zero.
Question 11.
Write the oxidation number of nitrogen in ammonium nitrate.
Answer:
NH4NO3
x + 4 × (+1) + (-1)= 0 × + 4 – 1= 0
x = – 4 + 1
x= – 3
∴ The oxidation number of nitrogen in ammonium nitrate is + 5 and -3.
![]()
Question 12.
Arrange the following in increasing order of oxidation states of Mn,
K2MnO4 , MnO2 , KMnO4
Answer:
Oxidation state of Mn in K2MnO4
1(2) + x + 4 (-2) = 0
2 + x – 8 = 0
x = 8 – 2
x = +6
Oxidation state of Mn in MnO2
x + 2 (-2) =0
x – 4 = 0
x= +4
Oxidation state of Mn in KMnO4
1 + x + 4 (-2) = 0
1 + x – 8 = 0
x – 7 = 0
x = + 7
∴ Increasing order of oxidation states of Mn,
MnO2 < K2MnO4 < KMnO4
![]()
Question 13.
What do you mean by redox reaction ?
Answer:
Redox reaction is the reaction in which both oxidation and reduction take place.
Question 14.
What is the oxidation number of Li i LiAlH4 ?
Answer:
x + 3 + 4 (-1) = 0
x + 3 – 4 = 0
x – 1 = 0
x = 1
∴ The oxidation number of Li : LiAlH4 is + 1.
Question 15.
Calculate the oxidation number of Fe in K4 [Fe(CN)6],
Answer.
4 (+1) + x + 6 (-1) – 0
4 + x – 6 = 0
x – 2 = 0
x= + 2
∴ The oxidation number Fe in K4 [Fe (CN)6] is + 2.
![]()
Question 16.
What is the maximum oxidation state of sulphur ?
Answer.
The maximum oxidation state of sulphur is +6.
RBSE Class 11 Chemistry Chapter 8 Short Answer Type Questions
Question 17.
How to balance a reaction taking place in basic medium by ion electron method ? Explain.
Answer:
There are following steps used-to balance a reaction taking place in basic medium by ion electron method :
Step I Write the skeletal equation and indicate the oxidation number of all the elements.
Step II Find out the species which are oxidised and which are reduced.
Step III Split the equation in two half reactions i.e. oxidation half reaction and reduction half reaction
Step IV Balance the two half reactions separately by using following steps :
- First of all, balance the atoms of the elements which have undergone a change in oxidation number
- Add electrons to whatever side in necessary to make up the difference in oxidation number in each half reaction.
- Balance change by adding OH–.
- Balance oxygen atoms by adding required number of H2O molecules to the side deficient in O-atoms.
- H-atoms are balanced by adding H2O molecules equal in number to the deficiency of H atoms and equal number of OH– ions are included in opposite side of the equation.
Step V The two half reactions are multiplied by suitable integers to balance the number of electrons. The half reaction, are then added up.
![]()
Question 18.
Explain the difference between oxidation state and valency of an element.
Answer:
| Oxidation state | Valency | ||
| (1) | In an atom or ion of a substance, the number of effective charge present on an element is called oxidation state of an element. | The valency of an element is a measure of its power with other atoms. | |
| (2) | The value of oxidation state may be positive, negative or zero. | Valency is always +ve. Inert gases have valency due to complete octet zero. | |
| (3) | In covalent compounds, oxidation state of an element is variable | In covalent compounds, the valency of an element is generally constant. |
Question 19.
2KI + Cl2 ➝ 2KCl + I2 , Identify the reducing agent in the reaction.
Answer:
2KI + Cl2 ➝ 2KCl + I2
Oxidation state of of I in KI => 1 + x = 0
x = -l
Oxidation state of I in I2= 0
Oxidation state of Cl in Cl2 = 0
Oxidation state of Cl in KC1 => 1 + x = 0
x = -l
![]()
Since, in this reaction Cl2 is reduced to KCl which is due to KI So, KI is reducing agent in the reaction.
![]()
Question 20.
Calculate the oxidation number :
(i) Mo in (NH4 )2Mo O4
(ii) Ni in [Ni (CN)4] 2-
Answer:
(i) Suppose oxidation number of Mo in (NH4 )2 MoO4 = x
2 (+1) + x+ 4 (-2) = 0
2 + x – 8 = 0
x – 6 = 0
x = + 6
(ii) Suppose, oxidation number of Ni in [Ni(CN)4 ]2- = + x
x + 4 (-1) = -2
x – 4 = – 2
x = -2 + 4 = +2
∴ Oxidation number of Ni in [Ni(CN)4 ]2 = + 2
Question 21.
Arrange the following metals in the order in which they displace each other from the solution of their salts – Cr, Cu, Mg, Zn, Fe, Al.
Answer:
More negative the value of standard electrode potential of a metal, more active will be the metal and a good reducing agents, This is the reason when a more active metal is placed in a solution of less active metal from the solution.
Metal ➝ Cr Cu Mg Zn Fe Al standard electrode
potential – 0.74, + 0. 34, – 2. 36, – 0.76, – 0.44, – 1.66, so, the order of metals in which they displace each other from the solution of their salts in :
Mg, Zn, Cr, Fe, Cu
(More active) (Least active)
![]()
Question 22.
Represent an electrochemical cell showing indirect redox reaction between Zn and ZnSO4 + Cu solution.
Answer:
Zn + CuSO4 ➝ ZnSO4 + Cu
The electrochemical cell of this reaction is represented as follows :
Anodic representation – Zn/ Zn2+(1M), which is written on the left side.
Cathodic representation- Cu2+ (1M)/Cu, which is written on the right side.
Salt bridge is represented by two line between the two half reactions.
![]()
Question 23.
Define oxidation and reduction :
(a) On the basis of electronic concept
(b) On the basic of oxidation number.
Answer:
(a) On the basic of electronic, oxidation may be defined as a reaction in which one or more electrons is lost by atom, ion or molecule.
The reduction may be defined as a reaction in which one or more electrons is gained by an atom, ion or molecule,
(b) On the basis of oxidation number, oxidation may be defined as a reaction in which the oxidation number of an element in an atom, molecule or ion increase. Reduction may be defined as a reaction in which the oxidation number of an element in an atom, ion or molecule decreases.
![]()
Question 24.
Identify oxidising agent and reducing agent in the following reactions :
H2O4 + O3 ➝ H2O + 2O2
2Na2S2O3 + I2 ➝ Na2S4O6 + 2Na I
Answer:
In a reaction
![]()
H2O2 is reduced to H2O and oxidised to oxygen. So, H2O2 acts as oxidising agent as well as reducing agent.
In a reaction
![]()
I2 acts as oxidising agent, which oxidise Na2S2O3 into Na2S4O6 and Na2S2O3 acts as reducing agent, which reduces I2 into NaI.
Question 25.
When an iron rod is dipped in silver nitrate solution, it became silverish. Explain the reason.
Answer:
The standard electrode potential of iron (Fe) is -0.44V and silver (Ag) is 0.80V. So, iron is more active metal which displaces silver from its solution, which gets deposited on iron rod so, iron rod becomes silverish when it is dipped in silver nitrate solution.
Fe + 2 Ag NO3 ➝ Fe(NO3 )2 + 2Ag
Question 26.
What is the oxidation number of oxygen in KO2 ?
Answer:
Lets the oxidation number of oxygen is KO2 = x
1+ 2 x = 0
2x = -1
x = -1/2
So, the oxidation number of oxygen on KO2 is – 1/2
![]()
Question 27.
During test of nitrate, a complex [Fe (H2O)5 (NO)(SO4 )] is formed. What the oxidation number of Fe in this complex ?
Answer:
[Fe(H2O)5(NO) (SO4)]
x + 0 + 0 – 2 = 0
x – 2 = 0
x= + 2
∴ Oxidation number of Fe in complex = + 2
Question 28.
Arrange the following in increasing order of oxidation states of iodine :
I2 , HI, HIO4, ICl
Answer:
Oxidation state of I in I2 => 0
Oxidation state of I in HI => 1 + x = 0, ⇒ x = -1
Oxidation state of I in HIO4 => 1 + x + 4(-2) = 0
1 + x – 8 = 0
x – 7 = 0
x = + 7
Oxidation state of I in ICl => x – 1 = 0
x =+l
Increasing order of oxidation state of iodine.
![]()
RBSE Class 11 Chemistry Chapter 8 Long Answer Type Questions
Question 29.
What is oxidation number ? How oxidation and reduction can be identified on the basis of change in oxidation number ? Write the step of balancing equation by oxidation number method.
Answer:
The number of effective charge present on an element of atom, ion or molecule in called oxidation number of that element. If oxidation number of an element on ion, atom or molecule increases, then oxidation takes place. Whereas if the oxidation number of element decreases, reduction takes place. The following steps are used for balancing equation by oxidation number method:
Step I First write the oxidation number of each element above its symbol.
Step II Identify the oxidation number of each element above its symbol.
Step III Identify the elements which undergo change in oxidation number.
Step IV Calculate the increases in oxidation number per atom. If more than .one atom of the same element is involved, find out the total number of increase or decrease in oxidation number.
Step V Equate the increase in oxidation number with decrease in oxidation number on reactant side by multiplying the formula of oxidizing and reducing agent.
Step VI Balance all other atoms except H and O.
![]()
Question 30.
What is standard electrode potential ? What is the importance of standard electrode potential in chemical reactions ? Explain with example.
Answer:
Standard electrode potential is defined as the potential of each electrode if the concentration of each taking part in reaction is unity at 1 atmospheric and 298K temperature. Importance of standard electrode potential in chemical reactions :
1. The reduction potential of lithium is minimum. It has highest capacity to lose electrons, hence, it is the strongest reducing agent. Whereas the reduction potential of fluoride is higher, it as higher capacity to accept electrons and thus strongest oxidising agent.
2. More negative the value of standard electrode potential of metal, more active will be the metal and a good reducing agent. This is the reason when a more active metal is placed in a solution of less active metal, it displaces the less active metal from the solution.
3. The metal in the top of activity series are good reducing agents therefore, their cations cannot be reduced by chemical metals. This is the reason why alkali metals, alkaline earth metals and aluminum etc. are obtained by electrolytic reduction method. The value of E° will be negative.
4. The metals placed above the hydrogen in activity series reduces hydrogen ion into hydrogen. This is why metals react with acids and liberate hydrogen gas.
Zn + 2H+ -> Zn2+ + H2
Mg + 2H+ -> Mg2+ +H2
5. Any non-metal displace the anion of the non-metal placed above it in the series from its solution.
Cl2 + 2Br– —> 2Cl– + Br2
6. The element present below in the series have more capacity to accept electrons as compared to lose electrons. They are good oxidising agents. For example, their cations oxidised H2 to H+ions
2Au+ + 3H2 —> 2Au + 6H+
7. Gold and Silver can be precipitated from their salt solutions by the metals more electronegative like zinc, aluminum etc.
2Na[Ag(CN2)]+ Zn -> Na2[Zn(CN)4 ] + 2Ag ↓
![]()
Question 31.
Balance the following reaction by oxidation number method and also identify oxidising agent.
(i) H2S + \(\mathrm{MnO}_{4}^{-}\) ➝ S + Mn2+ + H2O (Acidic medium)
Cl2O7 + H2O2 ➝ \(\mathrm{ClO}_{2}^{-}\) + O2(gas) (Basic medium)
Answer:
(i)(a) Write unbalanced equation,
![]()
(b) Oxidation number of Mn decreases from + 7 to +2. So, \(\mathrm{MnO}_{4}^{-}\) is reduced to Mn+2 and oxidation number of S increase from -2 to 0 so, H2S is oxidised to S. Hence, writing partial equation as,
![]()
Oxidation number decreases 5 (Reduction)
![]()
Oxidation number increases by 2 (Oxidation)
Multiplying equation 2 by 2 and equation 3 by 5, we get
\(2 \mathrm{MnO}_{4}^{-}\) ➝ 2Mn+2 … (4)
5H2S ➝ 5S …(5)
Adding equations 4 and 5, we get
2MnO4– + 5H2S ➝ 2Mn2+ + 5S … (6)
Change (-1) (0) (+4) (0)
(c) Addition of water molecules in the direction of deficiency of oxygen.
2MnO4 + 5H2 S ➝ 2Mn2+ + 5S + 2Mn2++5S + 8H2O
(d) To balance hydrogen atom, H+ions are added to the left hand side of the reaction.
5H2S + \(2 \mathrm{MnO}_{4}^{-}\) + 6H+ ➝ 5S + 2Mn2+ + 8H2O
This is balanced equation.
(ii) (a) Write unbalanced equation,
![]()
(b) Oxidation number of Cl decrases from +7 to +3, so Cl2O7 is reduced to \(\mathrm{ClO}_{2}^{-}\) and oxidation number of O increases from -1 to 0. So, H2O2 is oxidised to O2. Hence, writing partial equations,
![]()
Oxidation number decreases by 4 (Reduction)
![]()
Multiplying equation 2 by 1 and equation 3 by 4 and also balancing chlorine in ag (2)
Cl2O7 ➝ \(2 \mathrm{ClO}_{2}^{-}\) …(4)
4H2O2 ➝ 4O2 •••(5)
Adding equation (4) and (5), we get
C12O7 + 4H2O2 ➝ 2ClO2 + 4O2
Change (0) (0) (-1)0
(c) Addition of water molecular in the direction of deficiency of oxygen and addion of OH– ions to left hand side
Cl2O7 + 4H2O2 + 2OH– ➝ 2ClO2– + 4O2 + 5H2O
This is balanced equation.
Question 32.
Write the step of balancing equation by Ion Electron method and balance the following reactions :
(i) Al + NO3– ➝ Al(OH)4– + NH3 (Basic)
(ii) MnO4– + Br– ➝ Mn2+ + Br2 (Acidic)
(iii) Cr2O72- + Fe3+ ➝ Cr3+ + H2O + Fe3+(Acidic)
Answer:
(i) (a) The unbalanced equation is :
![]()
First half reaction
A1 ➝ Al(OH)4– (Oxidation)
(a) To balance oxygen atoms, add 4H2O to left hand side
Al + 4H2O ➝ Al(OH)4–
(b) To balance hydrogen atoms, add H+ to right side
A1 + 4 H2O➝ Al (OH)4– + 4H+
Since, reaction is in basic medium, OH– is added to left side
Al + 4H2O ➝ 4 Al(OH)4– + 4H2O
(c) To balance change, electrons are added to right side
Al + 4H2O+ 4OH– ➝ Al(OH)4– + 4H2O+3\(\overline{e}\) …(1)
![]()
(a) To balance oxygen atoms, add 3H2O to right side
NO3– ➝ NH3 + 3H2O
(b) NO3– + 9H2O ➝ NH3 + 3H2O+ 9 OH–
(c) To balance charge, add electron to left side
NO3–+ 9H2O+ 8\(\overline{e}\) ➝ NH3 + 3H2O+ 9OH– …(2)
Multiply eq. (1) By 8 and equation 2 by 3 and add, we get equation.
(ii) \(\mathrm{MnO}_{4}^{-}\) + Br– ➝ Mn2+ + Br2 (Acidic medium)
![]()
To balance oxygen atoms, H2O is added on right side.
\(\mathrm{MnO}_{4}^{-}\) ➝ Mn2+ + 4H2O
(b) To balance hydrogen, H+ ions are added to left side
\(\mathrm{MnO}_{4}^{-}\) + 8H+ ➝ Mn2+ + 4H2O
(c) To balance charge, electrons are added left side
\(\mathrm{MnO}_{4}^{-}\) + 8H+ + 5e– ➝ Mn2+ + 4 H2O …(1)
![]()
or 2Br– ➝ Br2
To balance charge, 2\(\overline{e}\) are added right hand side
2Br– ➝ Br2 + 2\(\overline{e}\) …(2)
Now adding the eq. (1) and (2)
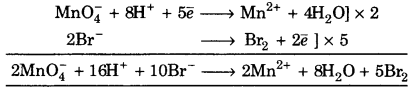
This is balanced equation.
(iii) (a) Balancing Cr, \(\mathrm{CrO}_{7}^{2-}\) ➝ 2Cr3+
(b) to balance oxygen atoms, 7H2O is added to the right side.
\(\mathrm{CrO}_{7}^{2-}\) ➝ Cr3+ + 7H2O
(c) To balance H atom H+ are added to the left side.
\(\mathrm{CrO}_{7}^{2-}\) + 14H+ ➝ 2Cr3+ + 7H2O
(d) To balance charge, electrons are added to the left
side. \(\mathrm{CrO}_{7}^{2-}\) + 14H+\(6 \overline{e}\) ➝ 2Cr3++7H2O …(1)
![]()
To balance charge, electrons are added to the right side.
Fe2+ ➝ Fe3+ + e– …(2)
Multiply equation (1) by 1 and equation 2 by 6 and add, we get.
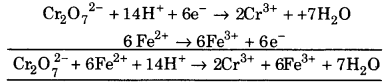
This is balanced equation.
![]()
Question 33.
Draw the diagram of Galvanic cell which represents the following reaction :
Zn + 2Ag+ ➝ Zn2++ 2 Ag
Answer the following question :
(a) Give equation for the reaction taking place at each electrode.
(b) In which direction electrons in external circuit flows ?
(c) Identify amode and cathode.
(d) If E° Zn2+/Zn = 0.76 an E° Ag+/ Ag = +0. 80 volt, then whether E° =: 1.56 volt ?
Ans.
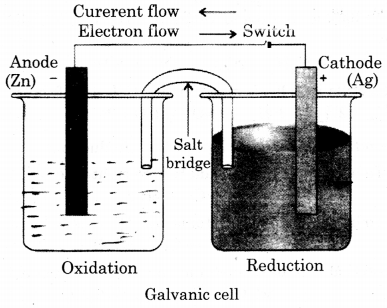
(a) Reaction taking place at anode :
Zn ➝> Zn2+ + 2e–
Reaction taking place at cathode-
2Ag+ + 2e– ➝ 2Ag
(b) Electrons in external circuit flow anode to cathode.
(c) Zn in anode and Ag in cathode.
(d) E°cell = E°cathode – E° anode
= E° Ag+ / Ag = E° Zn2+ / Zn
= 0. 80 – (- 0.76)
= 0.80 + 0.76 = 1.56 volt
Question 34.
What is electro chemical series ? The electrode potential of elements A, B, C and D are + 0. 79, -0. 74, + 1.08 and – 0.31 volts. Arrange them in increasing order of reactivity. To store 1 m HCl, which one aluminum or silver is suitable to use ? (E°Al3+ /Al = – 1.66V and E° Ag / Ag = +0.80V)
Answer:
Electro chemical series : The arrangement of elements in order of decreasing reduction potential values is called electro chemical series.
| Element | Electrode potential (V) |
| A | + 0.79 |
| B | -0.74 |
| C | +1.08 |
| D | -0.31 |
Increasing order of reactivity of these elements :
C < A < D < B
Least reactive Most reactive
The reactivity of elements depends on value of standard electrode potential more negative the value of standard electrode potential of element, more active will be the element.
Aluminum is suitable to store lm HCl because its standard electrode potential is more negative than silver.
![]()
Question 35.
Balance the following reaction using Ion electron method :
(i) MnO4– + SO32- -> Mn2+ + SO42-
(ii) Cr2O72- + H+ + I– -> Cr3++ H2O +I2 (Acidic medium)
(iii) Cl2 + OH– -> Cl– + ClO3–
(iv) N2H4 + ClO3– —> NO + Cl– (Basic medium)
Answer:
(i) MnO4– + SO32- —> Mn2+ + SO42- (Acidic medium)
![]()
(a) To balance oxygen atoms, addH2Oto right side.
MnO4– ➝ Mn2+ + 4H2O
(b) To balance hydrogen atoms, add H+ ions to left side.
MnO4– + 8H+ ➝ Mn2+ + 4H2O
(c) To balance charge, add electrons to left side.
MnO4–+ 8H+ + +5e– ➝ Mn2+ + 4H4O …(1)
![]()
(a) To balance oxygen atom, add H2O to left side.
SO32- + H2O ➝ SO42-
(b) To balance hydrogen atom, add H+ ions to right side.
SO32- + H2O ➝ SO42- + 2H+
(c) To balance charge, add electron to right side.
SO32- + H2O ➝ SO42- + 2H+ + 2e– …(2)
Multiply equation 1 by 2 and equation 2 by 5, and add, we get.
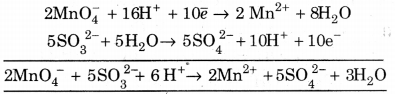
This is balanced equation.
(ii) Cr2O72- + H+ + I– ➝ Cr3+ + H2O+ I2
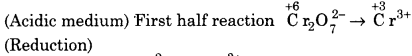
Balancing Cr, Cr2O72- ➝ 2Cr3+
(a) To balance oxygen atoms, add H2O to right side.
\(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) ➝ 2Cr3+ + 7H2O
(b) To balance hydrogen atoms, add H+ ions to left side.
\(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) + 14H+ ➝ 2Cr3+ + 7H2O
(c) To balance change, add electrons to left side.
\(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) + 14H+ + 6e–➝ 2Cr3+ +7H2O …(1)
![]()
(a) Balancing I, 2I– ➝ I2
(b) To balance charge, electron is added to right side.
2I– ➝ I2 + 2e– …(2)
Multiply equation (1) by 1 and equation 2 by 3 and add, we get
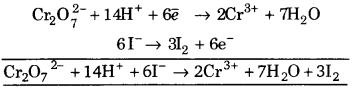
This is balanced equation.
(iii) Cl2 + OH– ➝ Cl– + ClO3–
![]()
Balancing Cl, Cl2 ➝ 2ClO3
(a) To balancing oxygen atoms, H20 as added to left side.
Cl2 +6H2O ➝ 2ClO3–
(b) To balance hydrogen atom, H+ are added to right side.
Cl2 + 6H2O ➝ 2CIO3– + 12H+
∴ Reaction is in basic medium OH– are added to left side
∴ Cl2+6H2O + 120H– ➝ 2ClO3– + 12H2O
(c) To balance charge, electrons are added to right side.
Cl2 +6H2O + 120H– ➝ 2CO3– + 12H2O + 10e– …(1)
![]()
Balancing Cl, Cl2 ➝ 2C1–
(a) To balance charge, electrons are added to left side.
Cl2 + 2e– ➝ 2Cl– …(2)
Multiply eq (1) by 1 and eq. (2) by 2 and add. we get
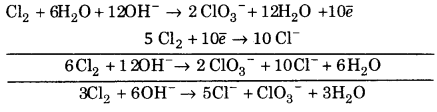
This is balanced equation.
(iv) N2H4 + ClO3– ➝ NO + Cl– (Basic medium)
![]()
Balancing N, N2H4 ➝ 2NO
(a) To balance oxygen atoms, add H2O to left side.
N2H4 + 2H2O ➝ 2NO
(b) To balance hydrogen atoms, add H+ ions to right side.
N2H4 + 2H2O ➝ 2NO+ 8 H+
∴ Reaction is in basic medium, add OH– to left side
N2H4 +2H2O + 80H+ ➝ 2NO + 8H2O + 8e– …(1)
![]()
(a) To balance O-atoms, add H2O to right side.
ClO3– ➝ Cl– +3H2O
(b) To balance hydrogen atoms, add H+ ions to left side.
ClO3– + 6H3+ ➝ Cl– + 3H2O
∴ Reaction is in basic medium , add OH– to right side
ClO3– + 6H3O ➝ Cl– + 3H2O + 60H–
(c) To balance charge, add electrons to left side.
ClO3– + 6H2O + 6e ➝ Cl– + 3H2O + 60H– …(2)
Multiply equation 1 by 3 equation 2 by 4 and add, we get.
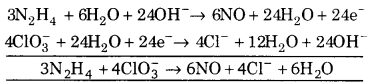
This is balanced equation.
![]()
Question 36.
Balance the following reaction using Ion Electron method,
(i) \(\mathrm{NO}_{3}^{-}\) + H+ + I– ➝ NO+ H2O + I2
(ii) CrO42- + SO32- + OH– ➝ CrO22- + \(\mathrm{SO}_{4}^{2-}\)
(iii) \(\mathrm{MnO}_{4}^{-}\) + Fe3O4 + OH– ➝ Fe2O3 + MnO2
(iv) P4 + OH– ➝ PH3 + \(\mathrm{HPO}_{2}^{-}\)
Answer:
(i) \(\mathrm{NO}_{3}^{-}\) + H+ +I– ➝ NO + H2O +I2
![]()
(a) To balance oxygen atoms add H2O to right side.
\(\mathrm{NO}_{3}^{-}\) ➝ NO + 2H2O
(b) To balance hydrogen atoms add H+ ions to left side.
\(\mathrm{NO}_{3}^{-}\) + 4H+ ➝ NO + 2H2O
(c) To balance charge, add electrons to left side.
\(\mathrm{NO}_{3}^{-}\) + 4H+ + 3e– ➝ NO + 2H2O …. (1)
![]()
Balancing I 2I– ➝ I2
(a) To balancing charge, electrons are added to right side.
2I– ➝ I2 + 2e–
Multiply eq (1) by 2 and eq. (2) by 3 and add, we get
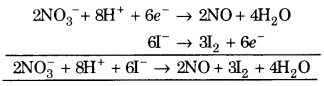
This is balanced equation.
(ii) \(\mathrm{CrO}_{4}^{2-}\) + SO32- + OH– ➝ \(\mathrm{CrO}_{2}^{2-}\) + SO42-
![]()
(a) To balance oxygen atoms add H2O to right side.
\(\mathrm{CrO}_{4}^{2-}\) ➝ \(\mathrm{CrO}_{2}^{2-}\) + 2H2O
(b) To balance hydrogen atoms add H+ ions to left side.
\(\mathrm{CrO}_{4}^{2-}\) + 4H+ ➝ \(\mathrm{CrO}_{2}^{2-}\) + 2H2O
(c) To balance charge, electrons are added to right side.
\(\mathrm{SO}_{3}^{2-}\) + 20H– ➝ \(\mathrm{SO}_{4}^{2-}\) + 2H2O + 2e– … (2)
Multiply eq 1 by 1 and eq. 2 by 2 and add, we get
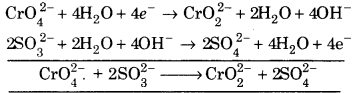
This is balanced equation.
(iii) \(\mathrm{MnO}_{4}^{-}\) + Fe3O4 + OH– ➝ Fe2O3 + MnO2
![]()
(a) To balance oxygen atoms add H2O to right side.
\(\mathrm{MnO}_{4}^{-}\) ➝ MnO2 + 2H2O
(b) To balance hydrogen atoms add H+ ions to left side.
\(\mathrm{MnO}_{4}^{-}\) + 4H+ ➝ MnO2 + 2H2O
∴ Reaction is in basic medium, OH– ions are added to right side
\(\mathrm{MnO}_{4}^{-}\) + 4H2O ➝ MnO2 + 2H2O + 4OH–
(c) To balance charge, electrons are added to right side.
\(\mathrm{MnO}_{4}^{-}\) + 4H2O + 3e– ➝ MnO2 + 2H2O + 4OH– … (1)
![]()
Balancing Fe, 2Fe3O4 ➝ 3Fe2O3
(a) To balance oxygen atoms, add H2O to left side.
2Fe3O4 + H2O ➝ 3Fe2O3
(b) To balance hydrogen atoms, add H+ ions to right side.
2Fe3O4 + H2O ➝ 3Fe2O3 + 2H2O
∴ Reaction is in basic medium, so add OH– ions to left side.
2Fe3O4 + H2O + 20H– ➝ 3Fe2O3 + 2H2O
(c) To balance charge, add electrons to right side,
2Fe3O4 + H2O + 20H– ➝ 3Fe2O3 + 2H2O + 2e– … (2)
Multiply eq. (1) by 2 and eq. (2) by 3 and add, we get
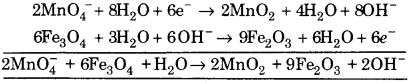
This is balanced equation.
(iv) P4 + OH– ➝ PH3 + HPO2–
![]()
Balancing P, P4 ➝ 4PH3
(a) To balance H-atoms, add H+ ions to left side.
P4 + 12H+ ➝ 4PH3
∴ Reaction is in basic medium, add OH” ions to right side.
P4 + 12H2O ➝ 4PH3 + 120H–
(b) To balance charge, electrons add to left side.
P4 +12H2O + 12e– ➝ 4PH3 + 120H– …( 1)
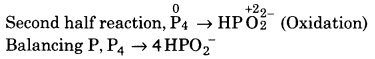
Balancing P, P4 ➝ 4HPO2–
(a) To balance hydrogen atoms, add H2O to left side.
P4 + 8H2O ➝ 4HPO2–
(b) To balance hydrogen atom, add H+ ions to right side.
P4 + 8H2O ➝ 4HPO2– + 12H+
∴ Reaction is in basic medium, add OH– to left side
P4 + 8H2O + 120H– ➝ 4 HPO2– +12 H2O
(c) To balance charge, electrons are added to right side.
P4 + 8H2O + 120H– ➝ 4HPO2– + 12H2O + 8e– …(2)
Multiply eq. (1) by 2 and eq (2) by 3, and add we get.
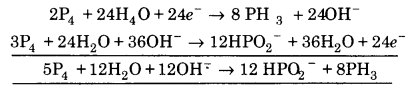
This is balanced equation.
![]()
Question 37.
Balance the following reactions using Ion Electron method :
(i) MnO4– + H2O2 ➝ MnO2 + O2 + OH–
(ii) As O33- + H2O + I2 ➝ ASO43- + H+ + I–
(iii) Cl2O7 + H2O2 ➝ ClO2– + O2 + H+
(iv) Cr2O72-+SO2 ➝ Cr3+ + SO42-
Answer:
(i) MnO4– + H2O2 ➝ MnO2 + O2 + OH–
![]()
(a) To balance oxygen atoms, add H2O to right side
MnO4– ➝ MnO2 + 2H2O
(b) To balance hydrogen atoms, add H+ ions to left side
MnO4– + 4H+ ➝ MnO2 + 2H2O
∵ The reaction is in basic medium, OH– are added to right side.
MnO4 + 4H2O ➝ MnO2 + 2H2O + 4OH–
(c) To balance charge, electrons are added to left side
MnO4– + 4H2O➝ MnO2 + 2H2O + 4OH– …(1)
![]()
(a) To balance hydrogen atoms, add H+ ions to right side
H2O2 ➝ O2 + 2H+
∵ Reaction is in basic medium, add OH– ions to left side
H2O2 + 20H– ➝ O2 + 2H2O
(b) To balance charge, electrons are added to right side
H2O2 + 20H– ➝ O2 + 2H2O + 2e– …(2)
Multiply equation 1 by 2 and equation 2 by 3, and add, we get
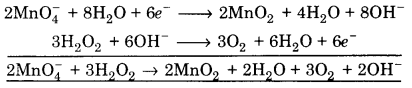
This is balanced equation.
(ii) AsO33- + H2O + I2 —> AsO43- + H+ + I–
![]()
(a) To balance oxygen atom, add H2O to left side
AsO33- + H2O ➝ AsO43-
(b) To balance hydrogen atoms, add H+ ions to right side
AsO33- + H2O ➝ AsO43- + 2H+
(c) To balance charge, electrons are added to right side
AsO33- + H2O ➝ AsO43- + 2H+ + 2e– …(1)
![]()
Balancing I, I2 ➝ 2I–
(a) To balance charge, electrons are added to leftside
I2 + 2e– ➝ 2I– …(2)
Add eq. (1) and (2)
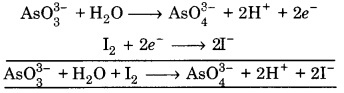
This is balanced equation.
(iii) Cl2O7 + H2O2 ➝ ClO2 + O2 + H+
![]()
Balancing Cl, Cl2O7 ➝ 2ClO2–
(a) To balance oxygen atoms, add H2O to right side
Cl2O7 ➝ 2ClO2– + 3H2O
(b) To balance hydrogen atoms add H+ ion to left side
Cl2O7 + 6H+ ➝ 2ClO2– + 3H2O
(c) To balance charge, electrons are added to left side
Cl2O7 + 6H+ + 8e– ➝ 2ClO2– + 3H2O …(1)
![]()
(a) To balance hydrogen atoms, add H+ ions to right side
H2O2 ➝ O2 + 2H+
(b) To balance charge, add electrons to right side
H2O2 ➝ O2 + 2H+ + 2e– …(2)
Multiply eq. (1) by 1 and eq. (2) by 4, and add, we get
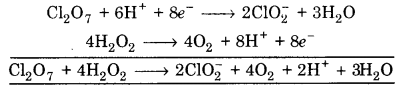
This is balanced equation.
(iv) Cr2O72- + H2O ➝ Cr3+ + SO42-
![]()
Balancing Cr, Cr2O72- ➝ 2Cr3+
(a) To balance oxygen atoms, add H2O to right side
Cr2O72- ➝ 2Cr3+ + 7H2O
(b) To balance hydrogen atoms add H+ ion to left side
Cr2O72- + 14H+ ➝ 2Cr3+ + 7H2O
(c) To balance charge, electrons are added to left side
Cr2O72- + 14H+ + 6e– ➝ 2Cr3+ + 7H2O …(1)
![]()
(a) To balance oxygen atoms, add H2O to left side
SO2 + 2H2O ➝ SO42-
(b) To balance hydrogen atoms add H+ ions to right side
SO2 + 2H2O ➝ SO42- + 4H+
(c) To balance charge, add electrons to right side.
SO2 + 2H2O ➝ SO42- + 4H+ + 2e– … (2)
Multiply eq. (1) by 1 and eq (2) by 3, and add, we get
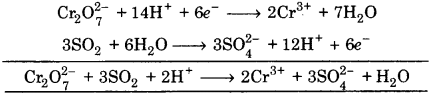
This is balanced equation.
![]()
Question 38.
Calculate oxidation number of the following compounds :
(i) Fe in FeSO4
(ii) Fe in Fe(CO)5
(iii) P in H3PO3
(iv) S in H2S2O7
(v) C in C12H22O11
(vi) P in NaH2PO2
Answer:
(i) Suppose oxidation number of Fe in FeSO4 = x
x + 1(-2) = 0
x – 2 = 0
x = +2
∴ Oxidation number of Fe FeSO4 = +2
(ii) Suppose, oxidation number of Fe in Fe(CO)5 = x
x + 5(0) = 0
x + 0 = 0
x = 0
∴ Oxidation number of Fe Fe(CO)5 = 0
(iii) Suppose, oxidation number of P in H3PO3 = x
3(+1) + x + 3(-2) = 0
3 + x – 6 = 0
x – 3 = 0
x = +3
∴ Oxidation number of P in H3PO3 = +3
(iv) Suppose, oxidation number of S in H2S2O7 = x
2(+1) + 2x + 7(-2) = 0
2 + 2x – 14 = 0
2x – 12 = 0
2x = 12
x\(=\frac{12}{2}\) = 6
∴ Oxidation number of S in H2S2O7 = +6
(v) Suppose, oxidation number of C in C12H22O11 = x
12x + 22(+1) + 11(-2) = 0
12x + 22 – 22 = 0
12x + 0 = 0
12x = 0
x = 0
∴ Oxidation number of C in C12H22O11 = 0
(vi) Suppose, oxidation number of C in P in NaH2PO2 = x
1(+1) + 2(+1) + x + 2(-2) = 0
1 + 2 + x – 4 = 0
x – 1 = 0
x = +1
∴ Oxidation number of C in P in NaH2PO2 = +1
![]()
Question 39.
Identify oxidising and reducing agent in the following:
(i) 3I2 + NaOH ➝ NaIO3 + 5NaI + 3H2O
(ii) AlCl3 + 3K ➝ Al + 3KCl
(iii) SO2 + 2H2S ➝ 3S + 2H2O
(iv) SnCl2 + 2FeCl3 ➝ SnCl4 + 2FeCl2
(v) H2O2 + H2O2 ➝ 2H2O + O2
Answer:
![]()
In this reaction, iodine (I2) is oxidised to NaIO3 and also reduced to Nal. So, it acts as both oxidising and reducing agent.
![]()
In this reaction, potassium (K) reduces AlCl3 to Al, So it acts as reducing agent and AlCl3 oxidises K to KCl, so it acts as oxidising agent
Oxidising agent = AlCl3
Reducing agent = K
![]()
In this reaction, SO2 oxidises H2S to S, so it acts as oxidising agent and H2S reduces SO2 to S, so it acts as reducing agent.
SO2 = Oxidising agent
H2S = Reducing agent
![]()
In this reaction, FeCl3 oxidises SnCl2 to SnCl4, so it acts as oxidising agent and SnCl2 reduces FeCl3 to FeCl2, so it acts as reducing agent.
FeCl3 = Oxidising agent
SnCl2 = Reducing agent
![]()
In this reaction, one molecule of H2O2 is oxidised to O2 and another molecule of H2O2 is reduced to H2O. So, H2O2 acts as both oxidising and reducing agent.
![]()
Question 40.
In which of the following reaction, H2O2 acts as oxidising agent and in which it acts as reducing agent?
(i) 2KI+ H2O2 ➝ 2KOH + I2
(ii) Cl2 + H2O2 ➝ 2HCl + O2
Answer:
![]()
In this reaction, H2O2 oxidises KI to I2 so it acts as oxidising agent.
![]()
In this reaction, H2O2 reduces Cl2 to HCl, so it acts as reducing agent.