Rajasthan Board RBSE Class 11 Chemistry Chapter 9 Hydrogen
RBSE Class 11 Chemistry Chapter 9 Text Book Questions
RBSE Class 11 Chemistry Chapter 9 Multiple Choice Questions
Question 1.
Pure hydrogen is obtained by electrolysis of
(a) Water containing Sulphuric acid
(b) Water containing NaOH
(c) Ba(OH)2
(d) Water containing KOH
Answer:
(c) Ba(OH)2
![]()
Question 2.
Molecular mass of heavy water is
(a) 10
(b) 12
(c) 18
(d) 20
Answer:
(d) 20
Question 3.
Which of the following is not a property of hydrogen peroxide?
(a) Reducing agent
(b) Oxidising agent
(c) Dehydration
(d) Bleaching agent
Answer:
(c) Dehydration
![]()
Question 4.
How ortho and para hydrogen are different?
(a) Number of protons
(b) Molecular mass
(c) Direction of rotation of electron
(d) Direction of rotation of proton
Answer:
(d) Direction of rotation of proton
Question 5.
Heavy water is
(a) D2O
(b) D2O2
(c) H2O
(d) H2O2
Answer:
(a) D2O
RBSE Class 11 Chemistry Chapter 9 Very Short Answer Type Questions
Question 6.
Who discovered hydrogen?
Answer:
Henry Cavendish discovered hydrogen in 1766.
Question 7.
In which group hydrogen is placed on the basis of electronic configuration?
Answer:
The electronic configuration of hydrogen is 1s1. On this basis, hydrogen is placed in group 1.
![]()
Question 8.
Write the name of isotopes of hydrogen and write the number of proton, electron and neutron in these.
Answer:
Isotopes of hydrogen
(a) Hydrogen (protium) \(_{1}^{1} \mathrm{H}\)
No. of protons = 1
No. of electrons = 1
No. of neutrons 1 – 1 = 0
(b) Deutrium \(_{1}^{2} \mathrm{H}\)
No. of protons = 1
No. of electrons = 1
No. of neutrons = 2 – 1 = 1
(c) Tritium \(_{1}^{3} \mathrm{H}\)
No. of protons = 1
No. of electrons = 1
No. of neutrons = 3 – 1 = 2.
Question 9.
What is ortho-hydrogen?
Answer:
The hydrogen in which rotation of proton or nucleus is in same direction is called ortho-hydrogen.
![]()
Question 10.
Which of the isotopes of hydrogen is radioactive?
Answer:
Tritium (\(_{1}^{3} \mathrm{H}\)) is radioactive isotope of hydrogen.
Question 11.
Helium is filled in balloons in place of hydrogen. Why?
Answer:
Helium is filled in balloons in place of hydrogen because helium is a noble gas and is not explosive in nature, while hydrogen is explosive in nature.
Question 12.
What is the oxidation number of oxygen in water?
Answer:
H2O Let, oxidation number of oxygen = x
2(+1) + x = 0
x + 2 = 0
x = – 2
![]()
Question 13.
What is the value of bond angle in water molecule?
Answer:
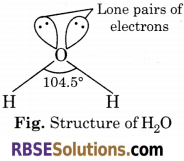
The value of bond angle in water molecule is 104.5°.
Question 14.
What is the reason of high boiling point of water?
Answer:
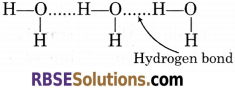
The high boiling point of water is due to presence of inter-molecular hydrogen bonding in water. High energy is required to break the bond.
Question 15.
At what temperature, density of water is maximum.
Answer:
At 4°C temperature, density of water is maximum.
![]()
Question 16.
Which gas is produced by reaction of calcium carbide on water?
Answer:
Acetylene gas is produced by reaction of calcium carbide on water
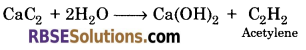
Question 17.
What is the reason of temporary hardness of water?
Answer:
Temporary hardness of water is due to the presence of bicarbonates of calcium and magnesium.
Question 18.
Write the chemical formula of calgon.
Answer:
The chemical formula of calgon is Na2[Na4(PO3)6].
Question 19.
What is the oxidation number of oxygen in hydrogen peroxide?
Answer:
Let, oxidation number of oxygen in H2O2 = x
2(+1) + 2x = 0
2 + 2x= 0
2x=-2
x = \(\frac{-2}{2}\) = -1
∴ The oxidation number of oxygen (O) in H2O2 is -1.
![]()
Question 20.
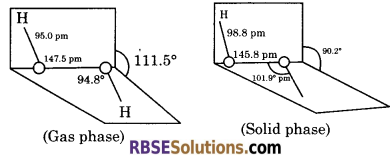
How is the structure of hydrogen peroxide?
Answer:
Hydrogen peroxide has a non-planar structure. It has an open book structure. The structure is shown as below
RBSE Class 11 Chemistry Chapter 9 Short Answer Type Questions
Question 21.
Write the similarities of hydrogen with alkali metals.
Answer:
There are following similarities of hydrogen with alkali metals
- Electronic Configuration : The electronic configuration of hydrogen is \(1 s^{1}\) which is similar to the outermost electronic configuration of alkali metals (\(n s^{1}\)). So, it is placed with alkali metals in group 1.
- Electropositive Character : Hydrogen can form unipositive ion by losing one electron like alkali metals.
e.g., H ➝ H+ + e–
Na ➝ Na+ + e– - Oxidation State : Hydrogen exhibits oxidation state of +1 ion the compounds of hydrogen which is similar to alkali metals.
- Valency : Valency of hydrogen is 1 like alkali metals.
- Reaction with Non-metals : Like alkali metals, hydrogen forms binary compounds with non-metals.
- Reducing nature : Hydrogen is a good reducing agent like alkali metals.
![]()
Question 22.
Write the similarities of hydrogen with halogens.
Answer:
There are following similarities of hydrogen with halogens :
(i) Electronic Configuration :
Electronic configuration of hydrogen = \(1 s^{1}\)
General electronic configuration of halogens = ns2np5
i. e., Hydrogen requires one electron to attain noble gas configuration. Similarly, halogens also require one electron to attain noble gas configuration.
(ii) Electronegative Nature : Hydrogen has tendency to attract electrons like halogens.
e.g., H + e– ➝ H– (hydride ion)
Cl + e– ➝ Cl– (chloride ion)
(iii) Oxidation number: In addition to oxidation state of +1, hydrogen shows oxidation state of -1 like halogens in some of its compounds
e.g., NaH (Oxidation state of H = – 1)
NaCl (Oxidation state of Cl = – 1)
(iv) Valency : Valency of hydrogen is 1 like halogens.
(v) Reaction with metals : hydrogen combines with metals to form binary compounds similar to halogens.
e.g., 2Na + H2 ➝ 2NaH
2Na + Cl2 ➝ 2NaCl
(vi) Atomicity Hydrogen exists in diatomic form (H2) like halogens (e.g., Cl2).
![]()
Question 23.
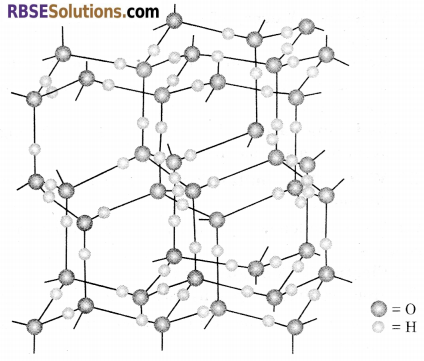
Why ice floats on water?
Answer:
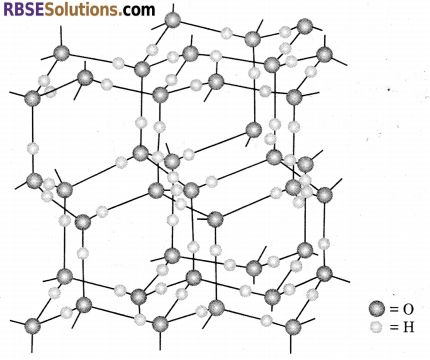
Ice is crystalline form of water. Ice has a highly ordered three dimensional structure containing hydrogen bond. X-ray studies show that each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm. One hydrogen atom lies in between each pair of oxygen atoms. So, each and every hydrogen atom is covalently bonded to an oxygen atom and linked to another oxygen atom by a hydrogen bond. This arrangement leads to a packing with large open spaces and results in lower density of ice than that of water. Hence, ice floats on water.
Question 24.
Explain the washing soda method for removal of permanent hardness of water.
Answer:
Washing soda reacts with soluble chlorides and sulphates of calcium and magnesium present in hard water to form insoluble carbonates which can be removed by filtration
CaCl2 + Na2CO3 ➝ CaCO3 ↓ + 2NaCl
MgCl2 + Na2CO3 ➝ MgCO3 ↓ + 2NaCl
CaSO4 + Na2CO3 ➝ CaCO3 ↓ + Na2SO4
MgSO4 + Na2CO3 ➝ MgCO3 ↓ + Na2SO4
Question 25.
Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Answer:
Hydrogen has an electronic configuration of \(1 \mathrm{s}^{1}\). Its outer shell is not complete. So, hydrogen forms covalent bond with another hydrogen atom which results in completely filled shell of electrons which is much more stable than half filled shell. Hence, hydrogen occurs in a diatomic form rather than in a monoatomic form under normal conditions.
![]()
Question 26.
Why the reactions of heavy water are slow?
Answer:
The O—D bond present in heavy water (D2O) is stronger than O—H bond in water (H2O). This is the reason that the reactions of heavy water are slow.
Question 27.
Write any four uses of hydrogen peroxide.
Answer:
There are following four uses of hydrogen peroxide:
- It is used as a hair bleach and as a mild disinfectant in daily life.
- It is used to manufacture chemicals like sodium perborate and per carbonate which are used in high quality detergents.
- It is used in the industries as a bleaching agent for textiles, paperpulp, leather etc.
- It is used to remove blackness of lead paintings in ancient times. Hydrogen peroxide changes black lead sulphide to white lead sulphate.
PbS + 4H2O ➝ PbSO4 + 4H2O
![]()
Question 28.
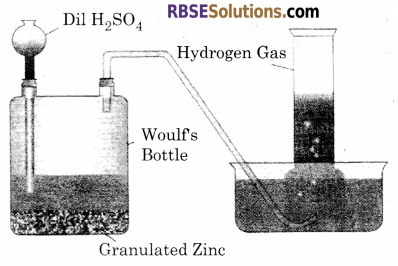
Explain the laboratory method for preparation of dihydrogen.
Answer:
Laboratory Method : In laboratory, dihydrogen is usually prepared by the reaction of granulated Zinc with dilute sulphuric acid. In this method granulated Zinc is taken in Woulfs bottle and Sulphuric acid is mixed produced with the help of a funnel. And the gas formed is collected in gas jar by water downward dislacement method.
Zn + H2SO4 ➝ ZnSO4 + H2 ↑
Question 29.
What is permutit?
Answer:
Permutit is also called zeolite. It is hydrated sodium alumino silicate. Its chemical (general) formula is Na2O . Al2O . xSiO2 . yH2O. It is used to remove the hardness of water.
Question 30.
What is heavy water?
Answer:
Heavy water is deuterium oxide (D2O). It was discovered by an American Scientist Harold C. Urey in 1932.
Question 31.
Why ancient lead paintings are washed with dilute solution of hydrogen peroxide?
Answer:
Ancient lead paintings are washed with dilute solution of hydrogen peroxide for removal of blackness of these paintaings. On exposure to lead paintings in atmosphere for a long time, lead oxide of paintings reacts with hydrogen sulphide to form lead sulphide. This causes the blackness of the paintings. Hydrogen peroxide changes black lead sulphide to white lead sulphate.
PbS + 4H2O2 ➝ PbSO4 + 4H2O
![]()
Question 32.
Explain the bleaching action of hydrogen peroxide.
Answer:
Hydrogen peroxide acts as a weak bleaching substance. Its bleaching action is due to its oxidation reaction
H2O2 ➝ H2O + [O]
Colouring matter + [O] ➝ Colourless matter
Question 33.
What is coal gasification?
Answer:
The process of producing ‘syngas’ from coal is called coal gasification. Syngas is also called synthetic gas. It is a mixture of CO and H2 which is used for the synthesis of methanol and a number of hydrocarbons

Question 34.
What are the reasons of temporary and permanent hardness of water?
Answer:
Temporary hardness of water is due to presence of bicarbonates of calcium and magnesium and permanent hardness of water is due to presence of chlorides and sulphates of calcium and magnesium.
![]()
Question 35.
What is known as storage of hydrogen and why?
Answer:
The storage of hydrogen is a key to enable technology for the advancement of hydrogen and fuel cell technologies in applications including stationary power, portable power and transportation. The calorific value of hydrogen is higher than petrol and other fuels.
RBSE Class 11 Chemistry Chapter 9 Long Answer Type Questions
Question 36.
It is justified to place hydrogen in a separate place in periodic table. Explain the statement.
Answer:
The position of hydrogen in periodic table is a subject of discussion, due to similarities and dissimilarities of hydrogen with alkali metals and halogens. It is difficult to assign a specific position to hydrogen. On the basis of electronic configuration, it is suitable to place hydrogen in group 1 of periodic table. But this is not right, hence due to its abnormal behaviour. It should be assigned a separate place in the periodic table. Keeping it walk other elements a drawback of periodic table.
![]()
Question 37.
What is hydride? How many types of hydrides are there? Explain with example.
Answer:
Hydrides The binary compounds of dihydrogen with other elements except noble gases are called hydrides.
Types of Hydrides
There are following types of hydrides :
1. Covalent of Molecular hydride : Dihydrogen forms molecular hydrides with most of the p-block elements. They consist of discrete covalent molecules which are held together by weak Vander Waal’s forces of attraction. They have low melting and boiling point. They are volatile in nature
e.g., CH4 , NH3, H2O and HF.
These are of three types on the basis of relative number of electrons and bonds in their Lewis structure :
- Electron-deficient hydride : Elements of group 13 will form electron-deficient hydrides. They act as Lewis acids, e.g., B2H6.
- Electron-precise hydride : All elements of group 14 form electron-precise hydrides, e.g., CH4.
- Electron-rich hydride : Elements of group 15-17 form such compounds. They act as Lewis bases, e.g., NH3.
2. Ionic or Saline hydrides These are stoichiometic compounds of dihydrogen formed with most of the s-block elements i.e., alkali and alkaline earth metals. These hydrides are crystalline, non-volatile and non-conducting in solid state. They have high melting and boiling points.
e.g., NaH, BeH2, MgH2 etc.
3. Metallic or Interstitial hydrides : These hydrides are formed by the reaction of dihydrogen with d-block and f-block elements. Due to small size of dihydrogen in these hydrides, hydrogen occupies interstices in the metal lattice producing distortion without any change in its type. So, these are known as interstitial hydrides. These are non-stoichiometric being deficient in hydrogen.
e.g., ScH2, LaH2, T2H2, CrH3 etc.
![]()
Question 38.
What do you understand by hardness of water ? Explain the permutit method for removal of permanent hardness of water with diagram.
Answer:
Hardness of Water : The capacity of water which restricts the formation of lather with soap is called hardness of water.
It is due to the presence of bicarbonates chlorides and sulphates of calcium and magnesium in water.
Permutit method for removal of permanent hardness of water This method is also called zeolite/ permutit process. Hydrated Sodium Aluminium silicate is zeolite/permutit. For the sake of simplicity, Sodium Aluminium Silicate (NaAlSiO4) can be written as NaZ. Water is softened by exchanging Ca2+ and Mg2+ ion with the help of zeolite. That’s why it is also called Zeolite or Permutit process. Zeolite is the hydrated silicate of Sodium and Aluminium. The general formula is (Na4O)x (Al4O3)y (SiO2 )z (H2O)n.
Let, Zeolite = Z = (Al2O3)y (SiO2 )z (H2O)n
CaSO4 + Na2.Z ➝ Ca Z + Na2SO4
MgCl2 + Na2 . Z ➝ Mg Z + 2NaCl
Na2Z(s) + M++(aq) ➝ MZ(s) + 2Na+(aq)
Where M = metal.
Zeolite is taken into the cement tank. Then hard raw water is entered into the inlet pipe. When water comes in contact with zeolite, water is softened. Zeolite is not used for every time while softening water. 10% NaCl is used through the Zeolites and the Zeolites are regenerated again.
MZ(s) + 2NaCl(ag) ➝ Na2Z(s) + MCl2(ag)
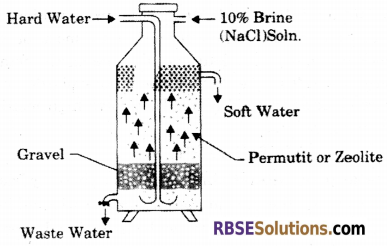
Question 39.
What do you mean by molecular association in water. Explain the structure of water with diagram. Explain the normal structure of ice.
Answer:
Molecular Association in Water : Molecular association’ in water is due to presence of inter- molecular hydrogen bonding
Structure of Water : A molecule of water consists of two hydrogen atoms joined to an oxygen atom by covalent bonds. The oxygen atom has six electrons in its outermost shell. The ‘s’ and ‘p orbital of the valence shell are sp3 hybridized to form four sp3 hybrid orbital oriented tetrahedrally around the oxygen atom. Two of the hybrid orbitals are singly occupied while the lone pairs of electrons occupy the other two. Each singly occupied sp3 orbital overlaps with the half filled 1s orbital of ‘H’ atom.
Hence, oxygen is bonded to the two hydrogen atoms by two O—H covalent bonds and two lone pairs of electrons are present on oxygen atom. Due to presence of lone pairs of electrons on the O-atom, the H—O—H bond angle is 104.5°, which is slightly less than the tetrahedral angle of 109° 28′. The structure of water molecule is an angular or bent structure.
Structure of Ice : Ice is crystalline form of water. It has highly ordered three-dimensional structure with hydrogen bonds. X-ray studies show that each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm. One hydrogen atom lies in between each pair of oxygen atoms. So, each and every hydrogen atom is covalently bonded to an oxygen atom and linked to another oxygen atom by a hydrogen bond. This arrangement leads to a packing with large open spaces.
![]()
Question 40.
Explain the following reaction with respect to hydrogen peroxide
(a) Oxidising and reducing nature
(b) Addition reactions
(c) Formation of peroxide
(d) Dissociation.
Answer:
(a) Oxidising and Reducing Nature : Hydrogen peroxide acts as a strong oxidising agent in both acidic and basic medium. In acidic medium, oxidation is slow while in basic medium, it is fast.
e.g., PbS(s) + 4H2O2 (aq) ➝ PbSO4 (s) + 4H2O(l) [in acidic medium]
MnSO4 + H2O2 + 2NaOH ➝ MnO2 + Na2SO4 + 2H2O [in basic medium] Hydrogen peroxide acts as a strong reducing agent for strong oxidising substances in both acidic and basic medium. The rate of reaction is slower in acidic medium as compared to basic medium.
e.g., K2Cr2O7 + 4H2SO4 + 3H2O2 ➝ K2SO4 + Cr2(SO4)3 + 7H2O + 3O2 [in acidic medium]
I2 + H2O2 + 20H– ➝ 2I– + 2H2O + O2 [in basic medium]
(b) Addition Reactions : Ethene reacts with hydrogen peroxide to form an addition product i. e., ethylene glycol.
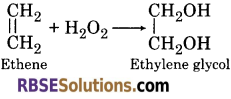
(c) Formation of Peroxide : Dehydrated hydrogen peroxide (acidic in nature) reacts with base to form salts (peroxide).
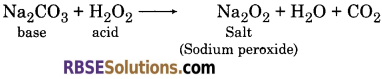
(d) Decomposition: Pure hydrogen peroxide is unstable. So, when it is placed in open air or on heating, it dissociates into H2O and oxygen.
2H2O2 ➝ 2H2O + O2
It is an exothermic reaction, which can be catalysed by Pt, Co, Au, Cu etc. It can be controlled by addition of small quantity of acid, alcohol or acetanilide.