Rajasthan Board RBSE Class 12 Biology Chapter 9 Enzymes
RBSE Class 12 Biology Chapter 9 Multiple Choice Questions
Question 1.
In what respect enzymes are different from catalysts?
(a) High diffusion rate
(b) Active at high temperature
(c) Protein nature
(d) Used in the reaction
Answer:
(c) Protein nature
Question 2.
The non-protein part of holoenzyme is called?
(a) Apoenzyme
(b) Co-factor
(c) Conjugated enzyme
(d) All the above
Answer:
(b) Co-factor
![]()
Question 3.
Which of the following statement is absolutely correct?
(a) All proteins are enzymes
(b) All enzymes are proteins
(c) Most enzymes are proteins
(d) None of the above
Answer:
(c) Most enzymes are proteins
Question 4.
Which enzyme was discovered first of all?
(a) Zymase
(b) Lipase
(c) Pepsin
(d) Isomerase
Answer:
(a) Zymase
Question 5.
Enzyme activity is affected by –
(a) pH only
(b) Substrate concentration
(c) only temperature
(d) All the above
Answer:
(d) All the above
Question 6.
Non-Competitive inhibitors are the substances which?
(a) Combine to the active sites of the enzyme
(b) Decrease the active sites of the enzyme
(c) Change the structural organisation of the enzyme
(d) Do not cause any change in the properties of an enzyme
Answer:
(c) Change the structural organisation of the enzyme
![]()
Question 7.
Which enzyme was obtained in a crystalline form first of all?
(a) Urease
(b) Catalase
(c) Amylase
(d) Aldolase
Answer:
(a) Urease
RBSE Class 12 Biology Chapter 9 Very Short Answer Questions
Question 1.
Who proposed lock and key theory and when was it proposed.
Answer:
Emil Fisher (1898).
Question 2.
The protein part and non-protein part of holoenzyme are called as?
Answer:
Protein part is named as apoenzyme and the non-protein part is called as co-factor.
Question 3.
Name a nonprotein enzyme.
Answer:
Ribozyme enzyme is made up of RNA and is the only non-proteinaceous enzyme known so far.
Question 4.
Define the prosthetic group.
Answer:
When the non-protein part of an enzyme is an organic substance and is firmly attached to the protein and can not be separated from it easily, it is called the prosthetic group.
RBSE Class 12 Biology Chapter 9 Short Answer Questions
Question 1.
What is co-enzyme? Give one example.
Answer:
When the non-protein part of the enzyme is an organic substance and it is closely attached to the protein part, which can dissociate from the protein part easily and can get attached again, it is called coenzyme.
Example: Co-A, NAD, NADP, FAD etc.
![]()
Question 2.
What do you understand by competitive inhibition? How this can be stopped.
Answer:
Some chemical substances which have structure closely similar to the substrate molecule, complete with the substrate in attaching with the active sites of the enzyme. Presence of such substances lowers the enzymatic activity. These substances are called competitive inhibitors and this inhibition is called competitive inhibition This type of inhibition can be stopped by increasing the concentration of substrate molecules.
Question 3.
Briefly explain the method of nomenclature of enzymes.
Answer:
There are several methods of naming enzymes. These are as follows:
- Most enzymes are named by adding suffix-case at the end of the name of substrate catalyzed by them. Example: Maltase, Sucrase, Urease.
- Many enzymes are named on the basis of the type of catalytic reaction governed by the enzyme. Example: Oxidase, Dehydrogenase etc.
- In the modern method of nomenclature, enzymes are named by adding suffix-case after both the name of the substrate on which they act and the type of catalytic reaction governed by them. Example: Succinate dehydrogenase.
- In some cases, traditional names of enzymes have been retained and are in practice. Example: Pepsin, Trypsin, Chymotrypsin.
- International Union of biochemistry has proposed systematic nomenclature in which some code number is given to each enzyme. In this nomenclature, substrate name, type of reaction catalyzed and some other information is also included.
Question 4.
How the rate of enzyme activity is accelerated.
Answer:
Enzymes are macromolecules and have high molecular weight. There are present numerous active sites on the surface of enzymes where the substrate molecules attach to form an unstable complex. This complex is called an enzyme-substrate complex. It breaks to release the product and enzyme.
By increasing the amount of enzyme, the rate of reaction increases, until the concentration of the substrate becomes a limiting factor. At this stage, the rate of reaction can be increased by increasing the concentration of substrate.
![]()
RBSE Class 12 Biology Chapter 9 Essay Type Questions
Question 1.
Describe the structure of the enzyme and explain their specific characteristics.
Answer:
- All enzymes are made up of proteins but all proteins are not enzymes.
- Many enzymes are made up exclusively of protein and such enzymes are called simple enzymes.
- In many enzymes, there is found a non-protein part associated with protein. Such enzymes are known as conjugated enzymes or holoenzymes.
- The protein part of the conjugated enzyme is called apoenzyme.
- The nonprotein part of the conjugated enzyme is called co-factor.
- The co-factors are of three types:
-
- Prosthetic group
- Co-enzyme
- Activator
Conjugated enzyme or Holoenzyme = Apoenzyme + Co-factor
1. Prosthetic Group:
- When the non-protein part attached with the apoenzyme is an organic compound and is firmly bonded with the protein part, it is called a prosthetic group.
- The prosthetic group can not be separated from the protein part (the apoenzyme) without denaturation of the protein.
Example: Cytochrome, Flavoprotein.
2. Co-enzyme:
- When the non-protein part is an organic compound and is only loosely attached with the protein part (the apoenzyme), it is called as co-enzyme.
- The co-enzyme can be easily separated from the protein part and can be easily attached again with it.
Example: Co-enzyme A, NAD, FAD, NADP etc.
3. Activator:
- When the non-protein part is an inorganic substance such as some metal ion or mineral ion, it is called as an activator.
- The main role of the activator is to form a bond between the enzyme and the substrate molecule Example: K, Cu, Mn, Fe Zn, Ca etc.
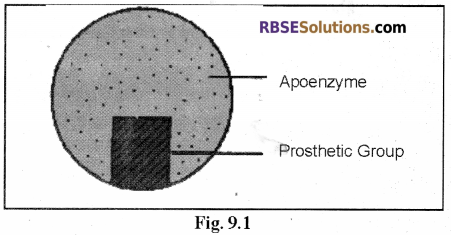
Note: The main part of the holoenzyme or conjugated enzyme is made up of protein and it is called apoenzyme. The size (dimension) of the protein molecule and the sequence of amino acids varies in different enzymes. - As the proteins are colloidal in nature, they have large surface area per unit volume.
- The protein part of the enzyme contains one or more specific sites called active sites. During the enzymatic activity, the substrate molecules attach at these active sites.
- In the holoenzyme of the conjugated enzyme, the enzymatic activity is brought about by the holoenzyme.
- The apoenzyme alone or the nonprotein part alone can not act as an enzyme.
Question 2.
Describe mechanisms of enzymatic action.
Answer:
All enzymes are macromolecules and have numerous specific areas called active sites on their surface.
- During the enzymatic activity, substrate molecules attach at these active sites and an unstable enzyme-substrate complex is formed.
- This enzyme-substrate complex dissociates to set free the product and the enzyme.
The mechanism of formation of the enzyme-substrate complex has been explained by two theories.
- Lock and key hypothesis
- Induced fit model theory
Formation of enzyme-substrate complex results in to lowering of the activation energy and hence the rate of reaction is accelerated.
![]()
Mode of Enzyme Action:
The mode of enzyme action depends upon the nature of the enzyme and the substrate molecule, and it can be understood by the following:
(1) Formation of Enzyme Substrate complex (ESC):
(2) Lowering of Activation energy.
(1) Formation of Enzyme Substrate Complex (ESC):
- In all enzyme governed reactions the enzyme and the substrate combine to form an unstable complex called enzyme-substrate complex. (ESC). This complex dissociates into the product and the enzyme.
Enzyme + Substrate → Enzyme substrate complex.
Enzyme substrate complex → Enzyme + Product(s).
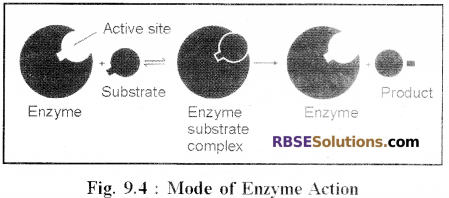
- On the surface of each enzyme, several specific areas are found. These are called active sites.
- The substrate molecules combine with the enzyme at these sites.
- When the substrate molecules combine at the active sites, they can easily react with each other forming the product.
- The product formed by formation of new bonds dissociates from the enzyme.
- The enzyme becomes free to bind with other molecules of the substrate and new enzyme-substrate molecule complex is formed.
The mechanism of formation of the enzyme-substrate complex and the property of specificity of the enzyme has been explained by the following theories.
1. Lock and Key Theory:
- This theory was postulated by Emil Fisher (1898).
- According to this theory, like a lock can be operated by it’s key only, similarly, a specific substrate having a specific structure only can bind with the specific active site present on the surface of a specific enzyme.
- The enzyme remains unchanged after the product is released.
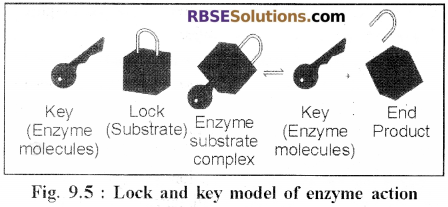
2. Induced Fit Model Theory:
- This theory has been proposed by Daniel Koshland (1966).
- According to this theory, the shape (structure) of active sites found on the surface of enzymes is not rigid but flexible.
- These active sites are initially not complementary to the substrate molecule, but as a specific substrate molecule comes in close proximity of the enzyme, it induces a change in the organisation of the active site and binds with it.
- In other words, it can be said that the configuration of active sites is not fixed or rigid as has been stipulated in the lock and key theory but is flexible and when a specific substance comes in contact, the active site undergoes a minor change and becomes complementary to the substrate molecule.
- Thus enzyme-substrate complex is formed between a specific substrate with a specific enzyme only.
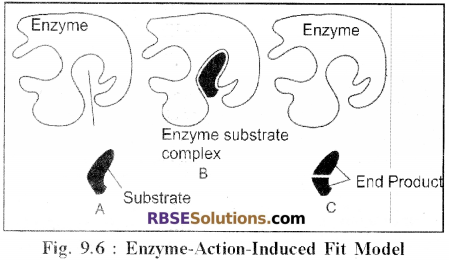
- This theory stipulates that there are two operative groups in the active sites one helps in binding of the substrate molecule and the other acts to convert the substrate into
the product.
![]()
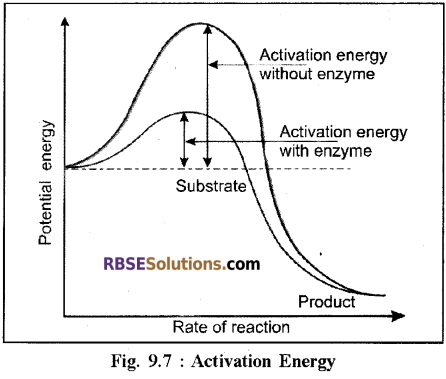
(2) Lowering of Activation Energy:
- All chemical compounds require an input of a certain amount of energy for initiating a chemical reaction. This energy is called activation energy.
- Enzymes have the ability to reduce the energy required for the activation of the molecule. • Hence in the presence of an enzyme, the reactant substrates are converted into a product with much lesser input of energy.
- On average the enzymes reduce the activation energy by approximately 65%.
- It is because of this that a reaction which takes place at a high temperature outside the cell takes place in the cell at atmospheric temperature with the help of enzyme.
Question 3.
Explain in details, classficiation of enzymes.
Answer:
International Union of Biochemistry (IUB) suggested the use of all the reactions and equations for the purpose of classification of enzymes and the Enzyme commission has classified all enzymes into six main divisions (categories).
1. Oxidoreductases
2. Transferases.
3. Hydrolases
4. Lyases
5. Isomerases
6. Ligases or Synthetases.
The six division are as follows:
1. Oxidoreductases:
All those enzymes which catalyze oxidation-reduction reactions are included in this category. They catalyze the removal or addition of hydrogen, oxygen or electrons from the substrate or to the substrate and thus bring about oxidation or reduction.
Note: On the basis of their activity these can be a divide into three subgroups:
(a) Oxidases: These bring about oxidation of the substrate by transferring hydrogen from the substrate molecule to oxygen.
Example: Cytochrome oxidase, Ascorbic acid oxidase etc.
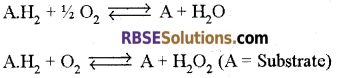
(b) Dehydrogenases: These enzymes bring about oxidation of substrate molecule by removal of hydrogen from the substrate as follows:
Example – Lactate dehydrogenase, Alcohol dehydrogenase.
![]()
(c)Reductases: These enzymes cause the addition of hydrogen or electron to the substrate and removal of oxygen.
![]()
2. Transferases:
These enzymes catalyze the transfer of a group (amino, phosphate, methyl, ketone etc.) from one molecule to another molecule.
Example: Transphosphatase, Transaminase.
![]()
3. Hydrolases:
These enzymes catalyze the addition or removal of water. They hydrolyse a variety of compounds and generally bring about cleavage of the substrate. This group includes digestive enzymes. Which breakdown macromolecules into smaller molecules.
Example: Carbohydrase, Amylase, Nuclease, Esterase etc.
Most hydrolytic reactions are reversible. These types of enzymes may also condense small molecules to form macromolecules. In such reactions, the water molecule is released. Example: Fumarase, Enolase etc.
4. Lyases:
These enzymes catalyze the removal of a group from their substrate molecule by breaking a special type of covalent bonds without hydrolysis.
Example: Aldolase.
5. Isomerases:
These enzymes catalyze intramolecular reorganisation in the substrate molecule and convert the substrate into its isomer.
Example: Phosphohexoisomerase.
6. Ligases or Synthetases:
These enzymes catalyze such reactions in which two compounds are linked together by forming covalent bonds between the two.
Example: Pyruvate carboxylase, Citrate synthetase.
Question 4.
What is enzyme inhibition? How many types of enzyme inhibition is known? Explain how the effect of inhibitors can be stopped.
Answer:
When an enzymatic activity is reduced or stopped by the effect of some chemicals others than the substrate molecules, the act is called enzyme inhibition. The chemicals or substances which bring about inhibition are called enzyme inhibitors.
Enzyme inhibition is of two types:
- Competitive inhibition
- Non-competitive inhibition
![]()
Enzyme inhibitors:
Some chemical substances inhibit enzyme activity. These substances are called enzyme
inhibitors and are of two types.
1. Competitive inhibitors:
These substances have structure closely similar to the structure of the substrate molecules. Hence these substances compete with the substrate in binding with the active site of an enzyme. This results in a decrease in enzyme activity.
Example: Malonic acid is a competitive inhibitor of succinic acid. The effect of this kind of inhibition can be overcome by increasing the concentration of substrate.
2. Non-competitive inhibitors:
Some substances do not show resemblance in structure with substrate molecule but attach with the active site of the enzyme permanently and bring about structural changes in their configuration. These inhibitors permanently block or destroy the active site and thus act as a poison. These are called non-competitive inhibitors or enzyme poisons. Example: \({ Pb }^{ ++ }\), \({ Hg }^{ ++ }\), \({ Ag }^{ ++ }\) etc.
Note: Cyanide causes denaturation of cytochrome oxidase enzyme which is used in respiration reactions and thus acts as a poison. The effect of non-competitive inhibitors can be overcome by increasing the concentration of the enzyme.