Rajasthan Board RBSE Class 12 Chemistry Chapter 13 Organic Compounds with Functional Group-Containing Nitrogen
RBSE Class 12 Chemistry Chapter 13 Text Book Questions
RBSE Class 12 Chemistry Chapter 13 Multiple Choice Questions
Question 1.
Which of the following is most basic?
(a) CH3NH2
(b) (CH3)2NH
(c) (CH3 )3N
(d) C6H5NH2
Question 2.
Hinsberg’s reagent is
(a) Benzene sulphonyl chloride
(b) Benzene sulphonic acid
(c) Benzene sulphonamide
(d) Phenyl isocyanide
Question 3.
C3H9N does not show
(a) Primary amine
(b) Quaternary salt
(c) Tertiary amine
(d) Secondary amine
Question 4.
The hybridisation of N atom in alkyl amine is
(a) sp2
(b) sp3
(c) sp
(d) sp3d
Question 5.
The molecular formula of a compound having mustard oil smell is
(a) RCN
(b) RNC
(c) RNCO
(d) RNCS
Question 6.
The formula for chloropicrin is
(a) C(NO2)Cl3
(b) CCl(NO2)3
(c) C(NO2)2Cl2
(d) None of these
Question 7.
Nitration of benzene gives nitrobenzene when HNO3 and H2SO4 take part in the reaction. Here HNO3 works as
(a) as a base
(b) as an acid
(c) a reducing agent
(d) as a catalyst
Question 8.
Benzene diazonium chloride reacts with X gives dye, here reactant X is
(a) C2H5OH
(b) C6H6
(c) C6H5NH2
(d) H2O
Question 9.
The formula of acetonitrile is
(a) CH3CN
(b) CH3COCN
(c) CH3CH2CN
(d) CNCH2COOH
Question 10.
The major product of the reaction of methylamine with Tilden reagent is
(a) CH3OH
(b) CH3CHO
(c) CH3Cl
(d) CH3COOH
Answers:
1. (b)
2. (a)
3. (b)
4. (b)
5. (d)
6. (a)
7. (a)
8. (c)
9. (a)
10. (a)
RBSE Class 12 Chemistry Chapter 13 Very Short Answer Type Questions
Question 1.
What is the reason that aromatic diazonium salt is more stable than aliphatic diazonium salt?
Answer:
Aromatic diazonium salt is more stable than aliphatic diazonium salt due to the resonance stability of aromatic diazonium salt.
Question 2.
Alkylamines are strong base than ammonia. Explain.
Answer:
Alkylamine is strongly basic than ammonia due to the electron releasing nature of the alkyl group.
Basicity α +I effect
Alkylamine having more + I effect than NH3.
Question 3.
Identify X and Y in the following reactions:
R R – CO NH2 \(\underrightarrow { { Br }_{ 2 }/{ NaOH } } \) X \(\underrightarrow { { CHCl }_{ 3 }/{ KOH } } \) Y
Answer:
X= R – NH2 (Alkylamine)
Y = RNC N (Alkyl isocyanide)
Question 4.
Identify A and B in the following reactions.
![]()
Answer:
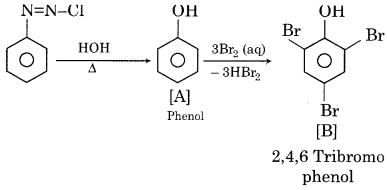
Question 5.
Dimethylamine is a stronger base than methylamine. Give reason.
Answer:
Dimethylamine is a stronger base than methylamine. Due to the more + I effect. + I effect for dimethyl group is more than the methyl group. So dimethylamine is the stronger base.
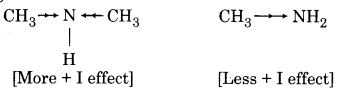
Question 6.
Give IUPAC name and structural formula of vinyl cyanide.
Answer:
Structural formula – CH2 = CH – CN
IUPAC Name – Prop – 2 – en – nitrile.
Question 7.
Write the equation of the Mendius reduction.
Answer:
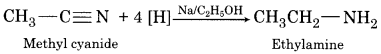
Question 8.
Write the equation for the conversion of aniline to phenyl isocyanide.
Answer:
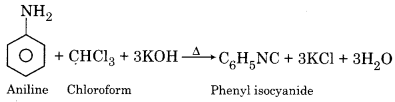
Question 9.
Write the reaction to obtain ethanol from Ethylamine.
Answer:
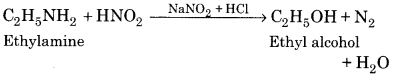
Question 10.
Give the structural formula of urea and write its IUPAC name.
Answer:

IUPAC name – Carbonyl diamide.
Question 11.
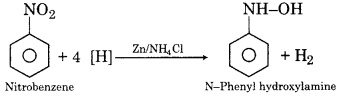
Write the reaction for the reduction of nitrobenzene in the presence of Zn + NH4Cl.
Answer:
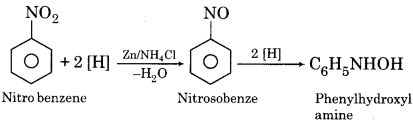
Question 12.
Complete the following reaction NH4CNO → K?
Answer:
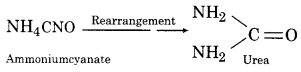
Question 13.
Give one equation to show the basic nature of ethanolamine.
Answer:
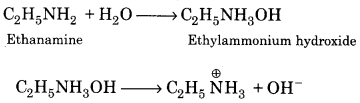
It is clear from the above equations that ethylamine is basic in nature.
Question 14.
The boiling point of primary amine is greater than tertiary amine. Why?
Answer:
The intermolecular association is maximum in primary amine. Tertiary amine does not have an intermolecular association because of the absence of hydrogen atom for hydrogen bond formation.
RBSE Class 12 Chemistry Chapter 13 Short Answer Type Questions
Question 1.
What is the biuret test for urea? Explain with the chemical equation.
Answer:
When urea is heated at 155°C, two molecules of urea react to release of NH3 and gives biuret, a white crystalline solid.
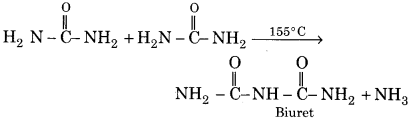
Above reaction is a test of urea because on adding an alkaline solution of copper sulphate to white solid biuret, violet colour is obtained.
Question 2.
Give the reaction of urea with:
- Formaldehyde
- Hydrazine
- Malonic acid
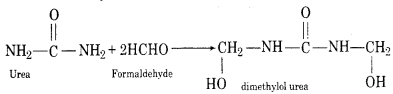
Answer:
1. Reaction with Formaldehyde: Urea reacts with formaldehyde and forms dimethyl urea.
2. Reaction with Hydrazine: Urea forms semicarbazide with hydrazine.
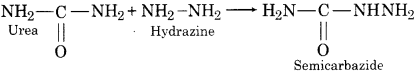
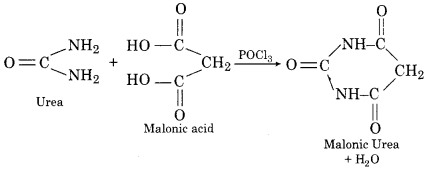
3. Reaction with Malonic Acid: Urea reacts with malonic acid to form barbituric urea or malonic urea.
Question 3.
Complete the following reactions:
R-X + KCN → ?
R-X + AgCN → ?
Answer:
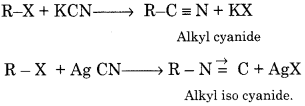
KCN is an ionic compound. On ionisation, it gives CN~ ion in which C and N both are nucleophiles but due to more nucleophilicity of negatively charged carbon, cyanide forms with KCN. Ag-C = N is a covalent compound. A lone pair of electrons is present on N atom of Ag-C=N which acts as a nucleophile. Hence, Ag- C N forms isocyanide.
Question 4.
Write the balanced chemical equation for the reduction of nitrobenzene in
- Basic medium
- Neutral medium
Answer:
1. Reduction of nitrobenzene in basic medium:
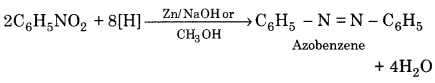
2. Reduction of nitrobenzene in neutral medium:
Question 5.
Write the aliphatic amines in increasing order of their basicity and give a short note on basicity.
Answer:
Due to the presence of unshared pair of electrons on nitrogen, amines are basic in nature. Aliphatic amines are a stronger base than ammonia due to +I effect of alkyl groups. Aromatic amines are weaker bases than ammonia due to the electron withdrawing nature of the aryl group.
The order of basicity of amines in the gaseous phase:
3° Amines > 2° Amines > 1° Amines > NH3
In aqueous solution basicity of amines:
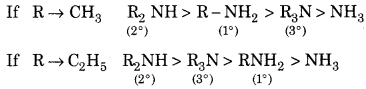
Question 6.
Write the structures of A, B and C in the following reactions:
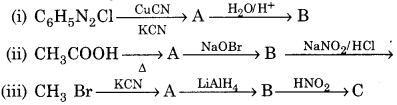
Answer:
(i)
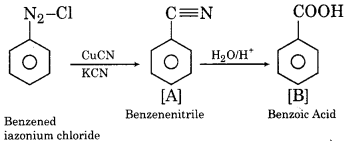
(ii)
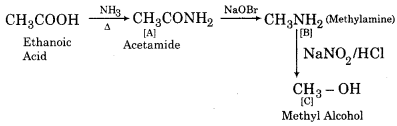
(iii)
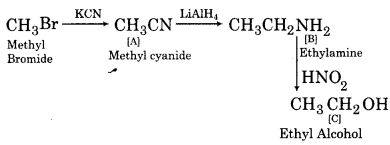
Question 7.
Write the reaction for hydrolysis of urea in different mediums.
Answer:
Hydrolysis of urea takes place with dilute acid, base and at high temperatures.
Basic medium:
NH2CONH2 + 2NaOH + 2HOH → 2NH4OH +Na2CO3
Acidic medium:
NH2CONH2 + 2HCl + HOH → 2NH4Cl + CO2
At High temperature:
NH2CONH2 + 2HNO2 → CO2 + 2N2+ 3H2O