Rajasthan Board RBSE Class 12 Chemistry Chapter 14 Bio-Molecules
RBSE Class 12 Chemistry Chapter 14 Text Book Questions
RBSE Class 12 Chemistry Chapter 14 Multiple Choice Questions
Question 1.
Which is known as the powerhouse of the cell?
(a) Golgi body
(b) Mitochondria
(c) Cytosome
(d) Ribosome
Question 2.
Which of the following is a disaccharide?
(a) Starch
(b) Fructose
(c) Lactose
(d) Cellulose
Question 3.
The product of hydrolysis of starch is
(a) Fructose
(b) Sucrose
(c) Maltose
(d) Glucose
Question 4.
The molecular formula of most common disaccharide is:
(a) (C6H12O6)6
(b) (C12H22O11)
(c) (C10H22O11)
(d) (C18H22O11)
Question 5.
Which of the following is not a reducing sugar?
(a) Glucose
(b) Fructose
(c) Sucrose
(d) Maltose
Question 6.
The product obtained after hydrolysis of protein in the presence of an enzyme is
(a) Amino acids
(b) Hydroxy acid
(c) Aromatic acids
(d) Dicarboxylic acid
Question 7.
The example of globular protein is
(a) Collagen
(b) Insulin
(c) Myosin
(d) Keratin
Question 8.
Alanine is an example of
(a) Alpha-amino acid
(b) B-fructose
(c) Gamma-lactose
(d) Delta-cellulose
Question 9.
Which of the following is a basic amino acid?
(a) Glycine
(b) Aspartic acid
(c) Lysine
(d) Glutamine
Question 10.
Enzymes are
(a) Carbohydrates
(b) Proteins
(c) Fats
(d) Salts
Question 11.
The conversion of protein to amino acids takes place by which of the following enzyme?
(a) Lipase
(b) Maltase
(c) Trypsin
(d) Renin
Question 12.
Chemical messengers are
(a) Hormones
(b) Enzymes
(c) Vitamins
(d) Nucleic acids
Question 13.
Number of the thyroid gland in humans is
(a) One
(b) Two
(c) Three
(d) Four
Question 14.
Growth hormone is secreted by
(a) Thyroid gland
(b) Pituitary gland
(c) Thymus gland
(d) Sex organs
Question 15.
Vitamin A deficiency causes
(a) Night blindness
(b) Scurvy
(c) Beriberi
(d) Anaemia
Question 16.
In nucleic acids, nucleotides are attached to one another by
(a) Hydrogen bond
(b) Peptide bond
(c) Phosphorous group
(d) Glycosidic bond
Question 17.
How many nucleotides sequence forms one codon in mRNA for an amino acid?
(a) One
(b) Two
(c) Three
(d) Four
Question 18.
DNA and RNA have chiral asymmetric carbon atom. The reason for their chirality is
(a) Asymmetric base
(b) D-sugar component
(c) L-sugar component
(d) Asymmetric phosphate ester units
Question 19.
In RNA, nitrogenous bases are
(a) Adenine and guanine and cytosine and thymine
(b) Adenine and guanine and thymine and cytosine
(c) Adenine and thymine and guanine and cytosine
(d) Adenine and guanine and uracil and cytosine
Question 20.
The sequence in nucleic acids is
(a) Base – sugar – phosphate
(b) Sugar – base – phosphate
(c) Phosphate – base – sugar
(d) Base – phosphate-sugar
Answers:
1. (b)
2. (c)
3. (d)
4. (b)
5. (c)
6. (a)
7. (b)
8. (a)
9. (c)
10. (b)
11. (c)
12. (a)
13. (a)
14. (b)
15. (a)
16. (c)
17. (c)
18. (d)
19. (a)
20. (a)
RBSE Class 12 Chemistry Chapter 14 Very Short Answer Type Questions
Question 1.
Write the chemical composition of the cell.
Answer:
Chemically a cell composed of carbohydrates, proteins, fats, lipids, minerals, water and various inorganic and organic substances.
Question 2.
What are monosaccharides?
Answer:
Monosaccharides:
A carbohydrate that cannot be hydrolysed further to give a simpler unit of polyhydroxy aldehyde and ketone is called a monosaccharide.
Example: glucose and fructose etc.
Question 3.
What are non- sugars?
Answer:
The carbohydrates having no sweet taste are called non-sugar.
For example starch, cellulose etc.
Question 4.
What is the main structural difference between starch and cellulose?
Answer:
The basic difference in starch and cellulose lies in the nature of the glucose molecules present. Starch consists of amylase and amylopectin. In both of them α – D (+) glucose molecules linked in C1 – C4 manner. In amylopectin, these linear chains are further linked in C1– C6 manner. In cellulose β – D (+) glucose molecules are linked to one another in a C1 – C4 manner. Thus, starch and cellulose have a different arrangement of the constituents.
Question 5.
Define essential and non-essential amino acids with examples.
Answer:
(i) Essential Amino acids:
Those amino acids which are to be supplied through diet are called essential amino acids. They cannot be synthesized by the body.
For example arginine, valine, histidine, etc.
(ii) Non-essential Amino Acids:
Those amino acids that can be synthesized by the body are called non-essential amino acids.
For example glycine, alanine, serine, cysteine etc.
Question 6.
What is the main function of enzymes?
Answer:
Enzymes catalyse biological reactions take place within the body.
Question 7.
Why are hormones known as ‘glandular juice’?
Answer:
Hormones are also known as ‘Glandular Juices’ because they are secreted by endocrine glands.
Question 8.
Which vitamin, is soluble in water?
Answer:
Vitamin B, C is soluble in water.
Question 9.
Write the name of nitrogenous bases found in DNA.
Answer;
Purines: Adenine (A), Guanine (G)
Pyrimidines: Cytosine (C), Thymine (T)
Question 10.
What are the main functions of nucleic acids?
Answer:
1. Nucleic acids play a vital role in the transmission of heredity characters.
2. Nucleic acids help in the biosynthesis of proteins.
RBSE Class 12 Chemistry Chapter 14 Short Answer Type Questions
Question 1.
Write the functions of carbohydrates.
Answer:
1. Carbohydrates are the main source of energy for a living being.
2. Carbohydrates are the major constituent of the plant body.
3. Deoxyribose and ribose sugars are the constituents of genetic material i.e., DNA & RNA.
4. Starch is used as food. Cellulose acts as the source of shelter and clothing.
Question 2.
Write any two methods for the preparation of glucose.
Answer:
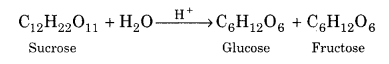
(i) Preparation of glucose from sucrose:
On boiling an aqueous solution of sucrose with dilute HCl or dilute H2SO4, glucose and fructose are formed in equimolar proportions.
(ii) Preparation of glucose from starch:
Starch, when boiled with dilute H2SO4 at 393 K under 2 to 3-atmosphere pressure, gives glucose.
![]()
Question 3.
What is Sickle Cell Anaemia?
Answer:
Sickle Cell Anaemia:
Haemoglobin, the molecule in the blood that carries oxygen consists of 574 amino acid units. Changing just one specific amino acid in the sequence results in defective haemoglobin found in patients suffering from sickle cell anaemia. In these patients the defective haemoglobin in RBCs precipitates causing the cells to sickle ie., the cells acquire a bent shape similar to a sickle, and some times cell bursts.
Normal Haemoglobin:
Val – His – Leu – Thr – Pro – Glu – Glu – Lys –
Sickle Cell Haemoglobin:
– Val – His – Leu – Thr – Pro – Val – Glu – Lys
The sickle cell haemoglobin contains valine (Val) in place of glutamic acid (Glu) present in normal haemoglobin.
Question 4.
Write the reactions of glucose with Fehling’s solution and Tollen’s reagent.
Answer:
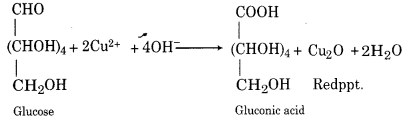
(i) Reaction with Fehling’s Solution:
A deep blue solution is obtained by mixing Fehling A (aqueous CuSO4 solution) and Fehling B (aqueous NaOH solution containing a small amount of Roschelle’s salt) in equal volumes. When heated with glucose then Fehling solution gives a red precipitate.
(ii) Reaction with Tollen’s Reagent:
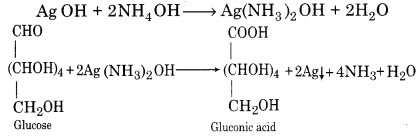
Tollen’s reagent is ammonical silver nitrate solution. When heated with glucose in a clean test tube, a shining mirror gets deposited at the bottom of the tube.
AgNO3 + NH4OH → AgOH + NH4NO3
Question 5.
Why are hormones known as “Chemical messengers”?
Answer:
Hormones are known as chemical messengers because they are secreted by glands and they work in the cells and the tissues away from their origin.
Question 6.
What is the isoelectric point of amino acids? Define.
Answer:
α-Amino acids behave as dipolar ion or as Zwitterion due to the transfer of a proton (H+) from carboxylic group to the amino group in the same molecule.
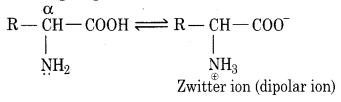
In acidic medium, the cation formed migrates towards cathode while in the basic medium anion migrates towards anode on passing an electric current. At a certain pH of the medium called an isoelectric point of an α-amino acid, the Zwitterion behaves as neutral and does not migrate to any electrode on passing current. The iso-electric points are in the pH range of 5.5 to 6.3.
Question 7.
Write one difference and one similarity between enzymes and hormones.
Answer;
Similarity:
Hormones and enzymes act as biocatalyst.
Difference:
Hormones act on glands or organs which are different from their origin organs or glands. These are released into the blood before use. Enzymes are not utilized in the reaction.
Question 8.
What do you understand by denaturation of protein? Explain.
Answer:
Denaturation of Proteins:
A protein present in the biological system with a unique three-dimensional structure and biological activity is known as the native protein. When a native protein is subjected to either some physical change or chemical change by taking part in certain chemical reactions which bring about a change in pH, this leads to denaturation of the native protein. The denaturation is due to the breaking of the H-bonds as a result of which the globules unfold and helix get uncoiled. The protein loses its biological activity on account of denaturation. Chemically denaturation does not change the primary structure of the protein and only brings about change in secondary and tertiary structures.
Question 9.
What is the genetic code?
Answer:
The sequence of amino acids in the protein synthesised is predetermined from the nucleotide base sequence of mRNA which in turn is correspondingly derived from the base sequence of DNA. The DNA sequence that codes for a specific protein or a polypeptide are called a gene. Thus every protein in a cell has a corresponding gene. The relation between the nucleotide triplets and the amino acid is called the genetic code.
Question 10.
Differentiate between primary and secondary structure of a protein.
| Primary Structure | Secondary Structure |
| Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and this sequence of amino acids is said to be the primary structure of that protein. | The secondary structure of a protein refers to the shape in which a long polypeptide chain can exit. These structures arise due to the regular folding to the backbone of the polypeptide chain due to H-bonding between -COOH and -NH- groups of the peptide bond. |
Question 11.
Explain mutarotation.
Answer:
Mutarotation:
The change in the specific rotation when the two anomeric forms are dissolved in water is known as mutarotation. Both the α and β forms of D (+) glucose are interconvertible through open chain structure. When α – form with specific rotation +112° is dissolved in water it slowly changes to β – form through the open chain structure. Similarly, when β – form with a specific rotation of +19° is dissolved in water it slowly changes to α – form in a similar manner. As a result of its specific rotation increase. Thus we conclude that both the forms in aqueous solution undergo a change in specific rotation and the change continues till the equilibrium, mixture acquires a specific rotation of about +52.7°. It has been established that the equilibrium contains about 36% of a-form and 63.5% of β – form with about 0.5% of open chain structure.

Question 12.
Write the name of the disease caused by the deficiency of Vitamin-B12 and Vitamin A. Also, write the source of these vitamins.
Answer:
| Vitamin | Deficiency Disease | Sources |
| B12 | Pernicious anaemia, inflammation of tongue and mouth | Milk, egg and animal tissues |
| A | Night blindness, stunted growth, xerosis (drying of the skin, xerophthalmia(cornea becomes opaque)) | Milk, butter, egg, fish and fish oil. It can also be synthesized in the body from carotenoids present in carrots, tomatoes, ripe mangoes etc. Carotenoids are the precursors of vitamin A. |
Question 13.
Write four differences between RNA and DNA.
Answer:
Differences between RNA and DNA
|
S.No |
RNA |
DNA |
|
1. |
The pentose sugar present in RNA is D-ribose. | The pentose sugar present in DNA is D-2-deoxyribose. |
|
2. |
RNA contains cytosine and uracil pyrimidine bases and guanine and adenine as purine bases. | DNA contains cytosine, and thymine as pyrimidine bases and guanine and adenine as purine bases. |
|
3. |
It is a single chain of a polynucleotide. | It is a double chain of a polynucleotide. |
|
4. |
It regulates protein synthesis. | It controls structure, metabolism, differentiation. Transfer the characters from our generation to the other. |
Question 14.
Write the Haworth structure of glucose and fructose.
Answer:
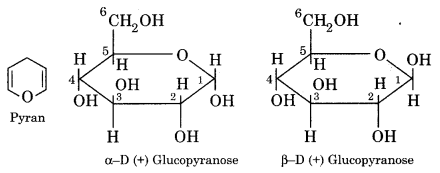
(i) Haworth Structure of Glucose:
The two anomeric forms (α and β) of D (+) glucose have been assigned pyranose ring structures by Haworth. Pyran is a six-membered heterocyclic ring containing an oxygen atom. The two cyclic structures for D (+) glucose are shown in the pyranose form
(ii) Haworth Structure of Fructose:
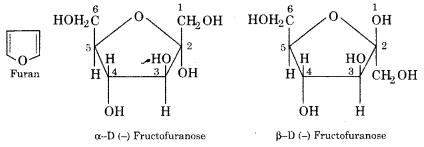
Haworth has purposed furanose ring structures for the two anomeric forms of D (-) fructose. Furan is a five-membered heterocyclic ring with an oxygen atom. This forms the basis of the two cyclic structures of D (-) fructose known as α – D (-) fructofuranose and β – D (-) fructofuranose.
Question 15.
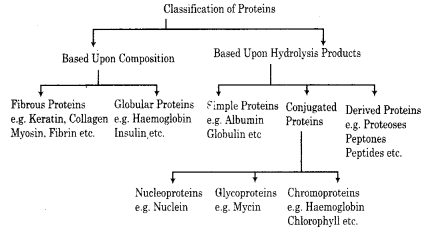
Define proteins and give their classification.
Answer:
Proteins:
A polypeptide with more than hundred amino acid residue having molecular mass higher than 10,000 u is called a protein.
RBSE Class 12 Chemistry Chapter 14 Long Answer Type Questions
Question 1.
Write the general chemical reactions of glucose.
Answer:
General chemical reactions of glucose can be grouped under two categories:
(a) Reactions due to alcoholic groups:
There are four secondary and one primary alcoholic groups present in a molecule of glucose. Some of the reactions of alcoholic groups are as follows:
(i) Acetylation:
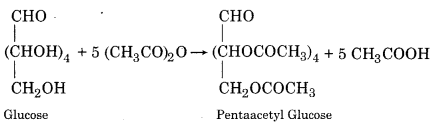
(ii) Methylation:
Glucose reacts with methyl alcohol in the presence of dry HCl gas forming methyl glucoside which is an ether.
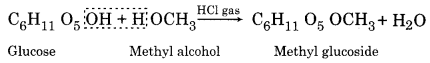
(b) Reactions due to aldehydic (-CHO) group
(i) Reduction:
When reduced with Na-Hg and water, the – CHO group in glucose is reduced to a primary alcoholic group to give sorbitol.
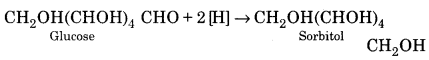
When reduction is carried with HI and red P, the product is n-hexane. This suggests that all six carbon atoms in glucose are linked to form a straight chain.
![]()
(ii) Oxidation:
With mild oxidising agent like Br2 water, glucose is oxidised to gluconic acid which is a carboxylic acid. This shows that the carbonyl group present in glucose is an aldehydic group.
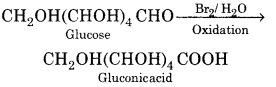
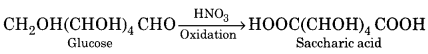
In case the oxidation is carried out with conc. HNO3 the primary alcoholic group is also oxidised to carboxyl group to form a dicarboxylic acid which is saccharic acid.
(iii) Rection with hydrogen cyanide:
Glucose forms a cyanohydrin derivative with hydrogen cyanide.
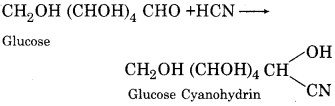
(iv) Reaction with hydroxylamine:
Glucose reacts with hydroxylamine to form an oxime.
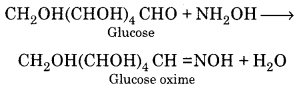
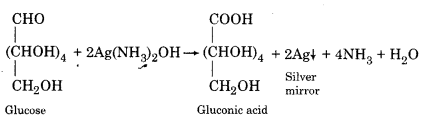
(v) Action with Tollen’s Reagent:
When Tollen’s reagent is heated with glucose in a clean test tube, a shining mirror gets deposited at the bottom of the test tube.
AgNO3 +NH4OH → AgOH+NH4NO3
AgOH + 2NH4OH → Ag(NH3)2OH + 2H2O
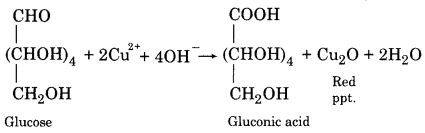
(vi) Action with Fehling’s Solution:
When heated with glucose, Fehling solution gives a red precipitate.
(vii) Reaction with Phenyl Hydrazine:
Glucose contains in it an aldehydic group and is expected to react with phenylhydrazine to form phenylhydrazone. However when the excess of phenylhydrazine is used the product formed is an osazone i.e., glucosamine.
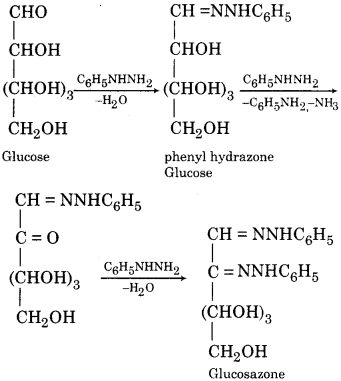
Question 2.
What are the main sources of cellulose and starch? Explain their structure in brief.
Answer:
(i) Cellulose (C6H10O5)n
Sources:
Cellulose occurs exclusively in plants and it is the most abundant organic substance in the plant kingdom. It is a predominant constituent of the cell wall of the plant cell. It is present in wood, cotton clothes, jute, cotton etc. Inwood, it is present 50%, in dry grasses, it is 40 – 45%, injure 60 – 65%, in cotton 90 – 95%, in cotton clothes 90% cellulose and rest in fats and waxes.
Structure:
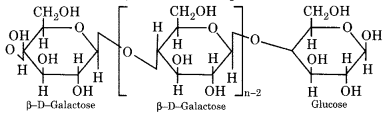
Cellulose is composed of β-D-glucose units linked by (1 → 4)glycosidic bonds. The X-ray analysis has shown that there are large linear chains of β – D(+) glucose molecules lying side by side in the form of bundles held together by H– bonding in the neighbouring hydroxyl groups. In each linear chain, the D(+) glucose unit are attached to each other by C1 to C4 bonds through β – glycosidic linkages as shown below.
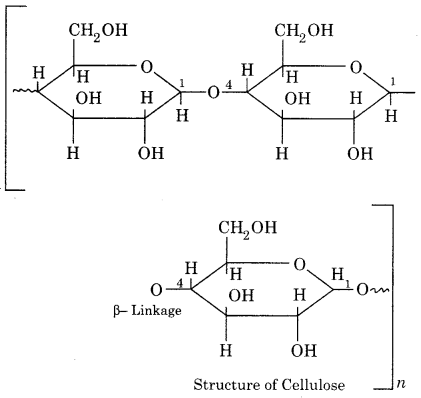
(ii) Starch (C6H10O5)n
Sources:
Starch is a major source of carbohydrates which are very much essential to the human body since they supply energy to the body. It occurs as granules mainly in seeds, fruits, tubers and also in the roots of plants. The chief commercial sources of starch are wheat, maize, rice, potatoes etc.
Structure:
Starch consists of two polysaccharide components. They are
(a) Amylose 15% – 20% soluble in water.
(b) Amylopectin (80% – 90%) insoluble in water.
![]()
Question 3.
What is the final product obtained by hydrolysis of the following:
(1) Fructose
(2) Lactose
(3) Sucrose
(4) Starch
(5) Maltose
(6) Cellulose
Answer:
(1) Fructose:
Being a monosaccharide it does not hydrolyse to is give simpler units at normal conditions.
(2) Lactose:
Upon hydrolysis with dilute mineral acid or with enzyme lactose gives equivalent amounts of β – D glucose and β – D galactose which is isomeric in nature.
![]()
(3) Sucrose:
Upon hydrolysis with dilute mineral acid or by enzyme sucrose form an equimolar mixture of D (+) glucose and D (-) fructose.
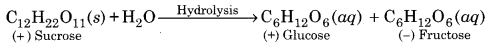
(4) Starch:
Upon hydrolysis with diastase enzyme starch gives maltose.
![]()
(5) Maltose:
Upon hydrolysis in presence of acid maltose gives two molecules of D – glucose.
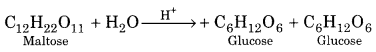
(6) Cellulose:
Upon hydrolysis at high pressure in the presence of dilute acid cellulose gives β – D-glucose.
![]()
Question 4.
Define proteins. Explain its hydrolysis. Explain the primary and secondary structures of proteins.
Answer:
The name “Protein” is given by Berzelius in 1938. The word protein is derived from the Greek word, “proteios” which means primary or of prime importance because proteins are the most important chemical substances which are necessary for growth, repair and development of life.
Proteins are found in all living cells. Main sources of proteins are milk, cheese, pulses, peanuts, fish, meat, etc. They occur in every part of the body and form the fundamental basis of structure and functions of life. In humans, hair, skin, haemoglobin, nails, enzymes and cells etc., are made of proteins.
Composition of Proteins:
Proteins are the high molecular mass complex biopolymers of amino acids and organic compounds that are most important for life. Chemically, all proteins are nitrogen-containing carbon compounds. All proteins contain the elements carbon, hydrogen, oxygen, nitrogen and sulphur some of these may also contain phosphorus, iodine and traces of metals like iron, copper, zinc and manganese. Amino acids are liberated when proteins are hydrolyzed. Proteins are the polymers of α – amino acids.
![]()
On partial hydrolysis of proteins, peptides of different molecular weight are obtained which on complete hydrolysis gives α – amino acids. This means that proteins are polypeptides of α – amino acids. Their molecular weight is more than 10,000. These are monodisperse natural polymers.
Question 5.
Write the functions of enzymes. Also, give their classification.
Answer:
Functions of enzymes:
| Where it is made? | Where does it work? | Enzyme | Substrate | Products |
| Salivary glands | Mouth | Amylase | Starch | Amino acids |
| Stomach cells | Stomach | Protease | Protein | Amino acids |
| Liver | Small intestine | Bile salts | Fat | Fat droplets |
| Pancreas | Small intestine | Amylase, Protease Lipase | Starch Protein Fat | Maltose amino acids glycerol and fatty acids |
| Small intestine | Small intestine | Maltase Protein | Maltase Protein | Glucose amino acids |
IUB (International Union of Biochemistry) in 1965 classified enzymes into six groups:
Classification of Enzymes
| S.No. | Group of enzyme | Reaction catalysed | Examples |
| 1. | Oxidoreductases | Transfer of hydrogen and oxygen atoms or electrons from one substrate to another. | Dehydrogenases |
| 2. | Transferases | Transfer of a specific group (a phosphate or methyl etc.) from one substrate to another. | Transaminase Kinases |
| 3. | Hydrolases | Hydrolysis of a substrate. | Esterases Digestive enzymes |
| 4. | Isomerases | Change of the molecular form of the substrate. | Phosphohexose isomerase, Fumarase |
| 5. | Lyases | Nonhydrolytic removal of a group or addition of a group to a substrate. | Decarboxylases Aldolases |
| 6. | Ligases(Synthetases) | Joining of two molecules by the formation of new bonds. | Citric acid synthetase |
Question 6.
Write the names and biological functions of hormones secreted by the pituitary gland and thyroid gland.
Answer:
| S.No. | Gland | Position | Hormone and their nature | Biological Functions |
| 1. | Pituitary | Beneath Brain | (i) Follicle stimulating hormone (FSH) (Protein) | Stimulates spermatogenesis in male, growth of ovarian follicle in a female. |
| (a) Anterior Lobe | (ii) Luteinising hormone (LH) (Protein) | Induces testosterone secretion in male. Ovulation and secretion of estrogen and progesterone in females. | ||
| (iii) Somatotrophin (STH) (Protein) | Regulates protein metabolism and growth of bones. Deficiency causes dwarfism, excesses produce gigantism in young and acromegaly in adults. | |||
| (iv) Prolactin (Protein) | Controls development of milk glands. | |||
| (v) Adrenocorticotrophin (ACTH) (Peptide) | Stimulates secretion of hormones of the adrenal cortex. | |||
| (vi) Thyrotrophin (TSH) (Protein) | Stimulates secretion of thyroid hormone. | |||
| (b) Intermediate lobe | (i) Melanocyte-stimulating hormone (MSH) (Peptide) | Controls skin colour. | ||
| (c) Posterior Lobe | (ii) Oxytocin (Peptide) | Regulate uterine contraction and release of milk. | ||
| 2. | Thyroid | Front of larynx | (i) Thyroxine | Regulate oxidation of food, excess causes exophthalmic goitre. |
| (ii) Triiodothyronine | Deficiency produces cretinism in children, myxoedema in adults. Also, control the working of kidneys. Deficiency also reduces urine output. | |||
| (iii) calcitonin (CT) (Peptide) | Controls calcium and phosphorus balance. |
Question 7.
What is vitamin B complex? Write the name of the disease caused by a deficiency of these vitamins.
Answer:
Vitamin B complex is a group of at least 13 vitamins usually named as B1, B2, B3 …etc. But to prevent confusion, their chemical names are now frequently used. The various members of the vitamin B complex are not related either chemically or physiologically yet they have many things in common. Diseases caused by the various vitamins of this group summarizes below:
| S.no | Vitamin complex | Deficiency diseases |
| 1. | Vitamin B1 | Beri-beri (paralysis of legs, normal weakness) and lack of appetite. |
| 2. | Vitamin B2 or riboflavin | Stunted growth, ulcers in mouth, skin dryness in corners of mouth and lips. |
| 3. | Vitamin B3 (it is made up of three substance pyridoxal, pyridoxine and pyridoxine) | Anaemia (lack of blood), nervous system and normal weakness body. |
| 4. | Vitamin B12 or Cyano-cobalamine | Pernicious Anaemia, loss of consciousness and swelling in the face. |
Question 8.
Explain the molecular structure of DNA.
Answer:
Molecular structure of DNA:
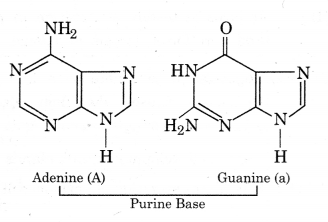
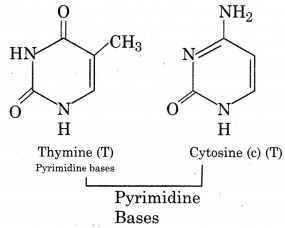
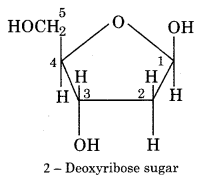
DNA molecule is made up of three units. These are nitrogenous bases:
1. Sugar and
2. Phosphoric acid
Nitrogenous bases:
DNA molecule contains two types of nitrogenous bases i.e., purine bases and pyrimidine bases.
1. Sugar:
2 – Deoxyribose sugar (pentose sugar) is present in the DNA molecule. It contains a five-membered heterocyclic ring and is generally called pentose sugar.
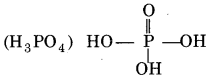
Arrangement of constituents in DNA molecule
A combination of base and Sugar is known as a nucleoside. Similarly, base, sugar phosphates form nucleotides. DNA molecules are the polymer of nucleotides.
Nitrogenous base + Sugar = Nucleoside
Nucleoside + Phosphoric acid = Nucleotide
Nucleotides = Polynucleotide
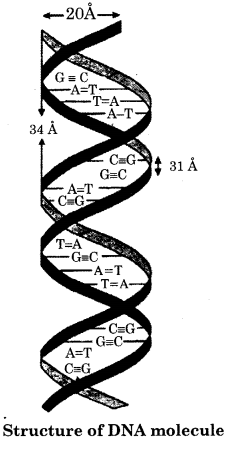
DNA is a macromolecule consisting of two polydeoxyribonucleotide chains. Each polydeoxyribonucleotide is formed by linkage of many deoxyribonucleotides through phosphodiester bonds. These bonds are present between two adjacent nucleotide components.
In DNA molecules Adenine base joined with Thymine base by a double bond and cytosine with guanine by triple bond ie., A=T, C=G.
Question 9.
How protein is synthesized by nucleic acid? Explain.
Answer:
Synthesis of protein:
Protein synthesis is another important function of nucleic acids. The process of protein synthesis is a very complex process. As stated earlier, 20 main acids combine in different ways to synthesise protein. Living cells have above 200 enzymes and more than 70 RNA take part in protein synthesis. Protein synthesis is done by different RNA molecules. DNA provides code for protein synthesis.
The synthesis of protein takes place in two steps:
1. Transcription:
Transmission of heredity information to RNA.
2. Translation:
Transmission of information from RNA to protein, the synthesis of protein from DNA can be represented as:
1. Transcription:
The process which leads to the synthesis of RNA is called transcription. It can also be defined as the process of copying genetic information from one strand of the DNA into RNA is termed as transcription. Three types of RNA takes part in protein synthesis (m-RNA, r-RNA and t-RNA) Which are synthesised by DNA.
Transcription involves the binding of RNA-polymerase at the promoter site on DNA. As it moves along (through structural gene), the DNA unwinds and one of the two strands acts as a template to synthesize a meaningful RNA and other strand act as non-coding. A complementary RNA strand is synthesized with A, U, C and G as bases. RNA synthesis is terminated when the RNA-polymerase falls off a terminator sequence on the DNA.
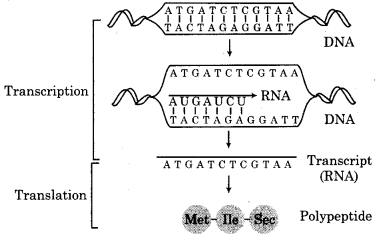
2. Translation:
This process is very complex. The formation of protein from m-RNA is known as translation. More than 100 macromolecules are used in this process such as m-RNA, t-RNA, ribosomes etc. It refers to the process of polymerisation of amino acids to form a polypeptide. The order and sequence of amino acids are defined by the sequence of bases in the m-RNA. The amino acids are joined by a bond which is known as a peptide bond. Protein synthesis takes place in the cytoplasm.
After transcription, m-RNA moves to a ribosome in cytoplasm form nucleus. r-RNA components of ribosomes. The base sequence in RNA is studied in three groups. This group of three nucleotide triplet is known as a codon. Each codon represents a particular amino acid. t-RNA has a specific sequence of bases at the ends which are complementary to the base sequence of m-RNA. At the other end of t-RNA, a specific amino acid is attached.
In ribosomes, amino acids retransferred from t-RNA to m-RNA. Those amino acids whose base sequence in m-RNA are bonded by m-RNA through peptide linkage. In this way, the polypeptide chain is formed and protein synthesis takes place. In proteins, a sequence of amino acids is determined by m-RNA and in m-RNA, a base sequence is determined by DNA. This means that the translation of amino acids takes place by DNA on ribosomes.