Rajasthan Board RBSE Class 12 Physics Chapter 15 Nuclear Physics
RBSE Class 12 Physics Chapter 15 Text Book Exercise with Answers
RBSE Class 12 Physics Chapter 15 Multiple Choice Type Questions
Question 1.
The radius of nucleus \(_{ 30 }^{ 64 }{ Zn }\) (in fm) is about:
(a) 1.2
(b) 2.4
(c) 4.8
(d) 3.7
Answer:
(c) 4.8
30Zn64
\(\frac{4}{3}\) πR3 ∝ A
R = R0A1/3
R = (1.2 fm)(64)1/3
R = (1.2 fm) (43)1/3
R = 4.8 fm
Question 2.
If the mass of isotope \(_{ 3 }^{ 7 }{ Li }\) is 7.016005 u and masses of H atom and a neutron are 1.007825 u and 1.008665 u respectively. The binding of Li nucleus is :
(a) 5.6 MeV
(b) 8.8 MeV
(c) 0.42 MeV
(d) 39.2 MeV
Answer:
(d) 39.2 MeV
Δm = [3 × 1.007825 u + 4 × 1.008665 u] – 7.016005 u
Δm = [3.023475 u + 4.03466 u] – 7.016005 u
Δm= [7.058135u – 7.016005u]
Δm = 0.042134 u
EB = Δmc2
EB = 0.04213 × \(931.5 \frac{\mathrm{MeV}}{c^{2}} c^{2}\)
EB = 39.24 Me V
Question 3.
If there are 1.024 × 1024 active atoms in a radioactive sample at any instant of time, then what would be the number of active atoms left after eight half lives?
(a) 1.024 × 1018
(b) 4.0 × 1021
(c) 6.4 × 1018
(d) 1.28 × 1019
Answer:
(b) 4.0 × 1021
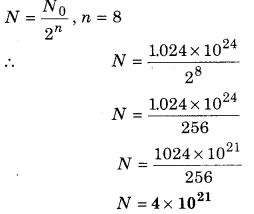
Question 4.
In an ancient sample of wood, the activity of 14C is 10 decays per second per gram in a sample whereas the activity of wood in fresh sample is 14.14 decays per second per gram. If the half life of 14C is 5700 years, then the age of the sample is about:
(a) 2850 years
(b) 4030 years
(c) 5700 years
(d) 8060 years
Answer:
(a) 2850 years
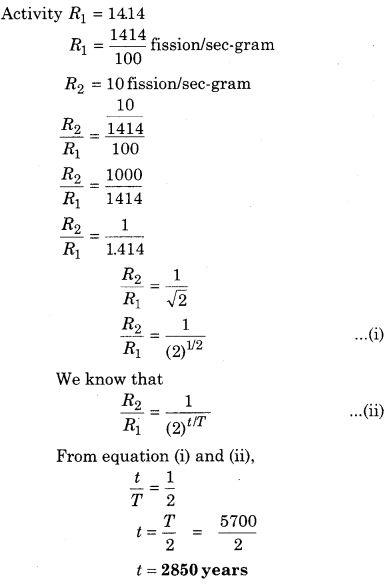
Question 5.
In the decay of \(_{ 92 }^{ 238 }{U } into _{ 82 }^{206}{ Pb }\) the number of emitted α and β particles are respectively :
(a) 8, 8
(b) 6, 6
(c) 6, 8
(d) 8, 6
Answer:
(d) 8, 6
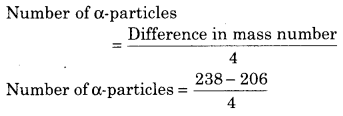
= \(\frac{32}{4}\)
= 8
Number of β-particles = 2 (Number of a-particles) – Difference in mass number
= 2 (8) – (92 – 82)
= 16 – 10
= 6
Question 6.
The binding energy per nucleon for a deuterium nucleus is 1.115 MeV. Mass defect for this nucleus is about:
(a) 2.23 u
(b) 0.0024 u
(c) 0.027 u
(d) Data is insufficient
Answer:
(b) 0.0024 u
For 1H2 mass number A = 2
Per nucleon binding energy of deuterium
\(\overline{E}_{b}\) = 1.115 MeV
Binding energy Eb = \(\overline{E}_{b}\) × A
Eb = 1.115 MeV × 2
Eb = 2.230 MeV
Mass number Δm = \(\frac{2.230}{931}\)
Δm = 0.0024 u
Question 7.
Two protons are at 10 A distance from each other. The nuclear force and electrostatic force between them is Fn and Fe respectively. Thus :
(a) Fn > > Fe
(b) Fe > > Fn
(c) Fn = Fe
(d) Fn is only a bit more than Fe–
Answer:
(b) Fe > > Fn
Fe >> Fn
Question 8.
The binding energies per nucleon for a deuteron and an α-particles are X1 and X2 respectively, then the free energy Q released in the fusion reaction, \(_{1}^{2} \mathrm{H}+_{1}^{2} \mathrm{H} \longrightarrow_{2}^{4} \mathrm{He}+Q\), is :
(a) 4 (X1 + X2)
(b) 4 (X1 – X2)
(c) 2 (X1 + X2)
(d) 2 (X2 – X1)
Answer:
(b) 4(X1 – X1)
1H2 + 1H2 → 2Hi4 + Q
Q = 2X1 + 2X1 – 4X2
Q = 4X1 – 4X2
Q = 4(X1 – X2)
Question 9.
The nucleus with highest binding energy per nucleon is :
![]()
Answer:
(d)
26Fe56 as maximum binding energy per nucleon.
Question 10.
A nuclear reactor of 40% efficiency has 1014 decays/second. If the energy obtained per fission is 250 MeV, then power of reactor is :
(a) 2 kW
(b) 4 kW
(c) 1.6 kW
(d) 3.2 kW
Answer:
(c) 1.6 kW
Per fission received energy = 250 MeV
= 250 × 106 × 1.6 × 10-19J
Number of fissions per second = 1014
∴ Per second received energy
= 250 × 106 × 1.6 × 10-19 × 1014
As efficiency is 40%, so output power
P = 250 × 106 × 1.6x 10-5 × \(\frac{40}{100}\)
= 1.6 × 103 watt = 1.6 kW
Question 11.
The production of emitted electrons in p decay is due to :
(a) Internal shells of atoms
(b) Free electrons in the nucleus
(c) Decomposition of neutron in nucleus
(d) Emitted photons in nucleus
Answer:
(c) Decomposition of neutron in nucleus
Due to decomposition of neutron in nucleus
Question 12.
In mean life :
(a) Half of the active nuclei decays
(b) More than half of the active nuclei decays
(c) Less than half are decayed
(d) All nuclei decays
Answer:
(b) More than half of the active nuclei decay
In mean life, more than half of the active nuclei decays
Question 13.
Which quantity related to nucleus is unchanged with increase in mass number?
(a) Mass
(b) Volume
(c) Binding energy
(d) Density
Answer:
(d) Density
Density does not depend upon mass number
Question 14.
Which of the following are electromagnetic waves?
(a) α rays
(b) β rays
(c) γ rays
(d) Cathode rays
Answer:
(c) γ rays
γ-rays are electromagnetic waves.
Question 15.
On absorbing energy 22Ne nucleus decays into a-particle and an unknown nucleus. The unknown nucleus is :
(a) Oxygen
(b) Boron
(c) Silicon
(d) Carbon
Answer:
(d) Carbon
22Ne → 2(2He4)+ 6C14
so, unknown nucleus is carbon.
RBSE Class 12 Physics Chapter 15 Very Short Answer Type Questions
Question 1.
What is the number of protons and neutrons in \(_{15}^{22} X\)?
Answer:
Comparing with ZXA
Z = 15 (number of protons)
A = 22
A – Z = 22-15=7 (number of neutrons)
Question 2.
Write equivalent energy (in MeV) of lu mass.
Answer:
931.5 MeV.
Question 3.
In what, does a nucleus transform its isotopes and isobars after β-decay?
Answer:
In isobaric nucleus because in β-decay one nucleon is transferred to second nucleon.
Question 4.
Out of which, α and βrays, has wide spectrum?
Answer:
α-particles.
Question 5.
On what type of chain reaction of fission is the nuclear reactor based?
Answer:
Based on controlled reaction.
Question 6.
Write the name of any one moderator.
Answer:
Graphite.
Question 7.
Write the relation between the half life of a radioactive substance, T and decay constant, λ.
Answer:
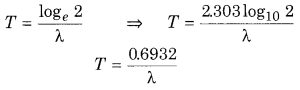
Question 8.
What is the SI unit of activity?
Answer:
1 Bacquerel = 1 disintegration/second.
Question 9.
How much amount in percent is left after four half lives of a radioactive substance?
Answer:
Number of active nucleus
N = \(\frac{N_{0}}{2^{n}}\)
N = \(\frac{N_{0}}{2^{4}}=\frac{N_{0}}{16} \times 100\)
N = 6.25%
Question 10.
Which nuclear reaction is responsible for energy production in Sun?
Answer:
Thermal nuclear fusion reaction.
Question 11.
A radioactive element whose mass number is 218 and atomic number is 84 emits β– particle. What would be the mass number and atomic number of element after decay?
Answer:
85X218 → 85Y218 + -1β0 + \(\overline{\mathbf{v}}\)
Atomic number of produced nucleus = 85
Mass number = 218
Question 12.
Is there any loss in mass number of nucleus after γ-decay?
Answer:
After γ-decay, there is no change in atomic number and mass number. But there is change of energy state.
Question 13.
Which out of iron or lead, is easier to take out an nucleon from?
Answer:
From lead it is easy. Per nucleon binding energy of lead is less than iron.
Question 14.
In a nuclear fission, the nucleus breaks into two nuclei of unequal mass. Which one of them (light or heavy) will have more kinetic energy?
Answer:
In nuclear fission, momentum remains conserved.
Ek = \(\frac{p^{2}}{2 m}\)
∴ Ek ∝ \(\frac{1}{m}\)
∴ There will be more K.E. of light nucleus.
Question 15.
If the nucleons of a nucleus are divided, then total mass increases. Where does this mass come from?
Answer:
This mass is received from the binding energy of the nucleus.
RBSE Class 12 Physics Chapter 15 Short Answer Type Questions
Question 1.
In a hydrogen molecule, there are two protons and two electrons. In the behaviour of hydrogen molecule, the nuclear force between these protons is always neglected. Why?
Answer:
The distance between molecules is of the order of Å, while nuclear forces act in distance of fermi order.
Question 2.
A student says that a heavy form (isotope) of hydrogen decomposes by α-decay. How would you react?
Answer:
Inα-decay, the daughter nucleus has atomic number less by 2. For hydrogen isotope this is not possible.
Question 3.
Define the unified atomic mass unit (u).
Answer:
Atomic Mass Unit
Atomic and nuclear masses are the order of 10-25kg to 10-27 kg. In real, it is not convenient to use such small units. So, they are represented by another small unit called unified atomic mass unit and it is denoted by the symbol u (earlier this unit was called atomic mass unit and symbol was a.m.u.). This unit is selected so that mass of the \(_{6}^{12} \mathrm{C}\) atom (and not the
nucleus) is 12 u.
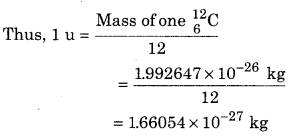
It is noted that the atomic mass represents the mass of neutral atoms and not the mass of its nucleus. This means that the mass of an atom always include the mass of its Z electrons. The measurement of atomic mass is done by mass spectrograph. On representing the atomic mass of many elements in atomic mass unit (u), they are close to the whole multiplies of the mass of hydrogen atom but there are few exceptions, like the atomic mass of chlorine is 35.46 u.
On using the famous mass energy equivalent relation of Einstein (E = mc2), we can determine the equivalent energy of mass of 1 u which calculated as :
m = 1 u = 1.66050 × 10-27 kg
∴ Equivalent energy
E = (1u) c2
E = (1.6605 × 10-27) (2.9979 × 108)2 kgm2/s2
= 1.4924 × 10-10 J = \(\frac{1.4924 \times 10^{-10}}{1.602 \times 10^{-19}} \mathrm{eV}\)
E = 931.5 MeV
This means that we can write u as 931.5MeV/c2 and can determine equivalent energy of mass and mass difference in u and vice-versa. Table 15.1 describes the mass of proton, neutron, electron and mass of hydrogen atom in different mass units.
Question 4.
Explain nuclear mass defect.
Answer:
We have read that the entire mass of the atom and positive charge is concentrated in the nucleus and nucleus is composed by the protons and neutrons. We also know how much protons and neutrons occur in the nucleus therefore, the expected mass of a nucleus can be determined by the calculation. The actual mass of nucleus can also be determined by mass spectrograph. It is found that the actual mass of any nucleus is always less than the expected mass obtained by its nucleons calculation. This difference of mass is called mass defect. Similarly,
Mass defect = Mass of nucleus obtained by the calculation — Actual mass of nucleus
or Δm = mc – ma
Where calculated mass is represented by mc and actual mass is represented by ma.
∴ Δm = [Mass of protons + mass of neutrons] – Actual mass of nucleus
or Δm = [Zmp +(A – Z) mn] – m …………… (1)
Where Z is atomic number, A’mass number, mp mass of proton, mn mass of neutron and m is the actual mass.
Question 5.
Define radioactivity.
Answer:
Radioactivity
In the beginning of this chapter, it was mentioned that Henry Becquerel discovered radioactivity in 1896. Heavy elements like uranium, thorium, etc. particles or radiations on their own and undergoes a decay. In this process of decay, new atoms (elements) are formed that themselves exhibit radioactivity and this process continues till a stable element is formed.
The discovery of radioactivity by Henry Becquerel was purely accidental. He observed that when uranium salt-crystals or uranium-potassium sulphate were illuminated with visible light, they emitted some invisible radiations that blackened the photographic plate placed near it which was covered by a light resistant cover. After this, scientists Marie Curie and Pierre Curie discovered the new elements radium and polonium from the pitch blend are which were more radioactive than uranium. Through the experiments, Rutherford showed that radioactive radiations are of three type which he called alpha, beta and gamma rays. The experiments performed later showed that-α (alpha) rays are helium nuclei, β (beta) rays are electrons or positrons and γ (gamma) rays are photons of high energy. Experiments also showed that the radioactivity is a result of decay of unstable nuclei. This way, we can say that radioactivity is a nuclear phenomenon. Some important facts related to radioactivity are as follows :
(i) External conditions like pressure temperature and state of radioactive substance (solid, liquid or gas) has no effect on radioactivity. Radioactivity is also unaffected by the chemical reactions or chemical combinations (like uranium or any of its compounds is radioactive). As there is contribution of only the outermost electrons in a chemical combination. Thus, the electronic configuration of atoms is not related to the radioactivity. Also, α-rays, β-particles of very high energy or emission of γ-ray photons is not possible from the emission of the outer part of atom. Thus, radioactivity is purely a nuclear phenomenon.
(ii) In the radioactive decay of nuclei, with the laws of conservation of mass, energy, charge linear momentum, angular momentum and the conservation of number of nucleons should be followed.
(iii) A nucleus say X is unstable for the decay of α and β if its mass is more than the sum of its product constituents.
(iv) In the process of radioactive decay, emission energy per atom is the order of few MeV where as it of the order of few eV for chemical reactions.
Question 6.
Explain Rutherford-Soddy law.
Answer:
Rutherford-Soddy Law of Radioactive Decay
In 1902, Rutherford and Soddy studied the disintegration of many radioactive substances and found the following conclusions regarding radioactive decay known as Rutherford and Soddy Rules. According to :
- Radioactivity is a nuclear phenomenon and the rate of emission of radioactive ray cannot be controlled by physical or chemical process that mean neither can it be extended nor can it be reduced.
- The nature of the disintegration of radioactive substance is statistical, this is, it is very difficult to say which nucleus will be disintegrated and which particle will emit α, β and γ With the emission of α , β and γ rays in the process of disintegration one element change into another new element, its chemical and radioactive qualities are completely new.
- At any time the rate of decay of radioactive atom is proportional to the number of atoms present at that time.
Let the number of atoms present at any given time t is N and at the time t + Δt this number decreases N – ΔN to its value then, the rate of decay of atoms is \(-\frac{\Delta N}{\Delta t}\). Therefore, according to the law of Rutherford and Soddy.
If at the time Δt the nucleus ΔN will be disintegrated then the rate of disintegration,
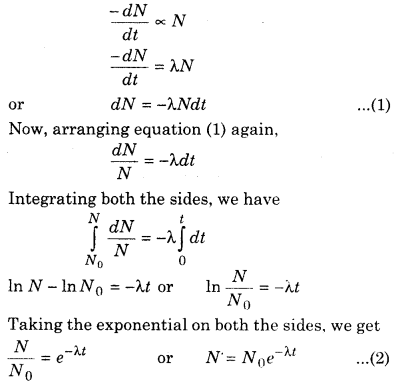
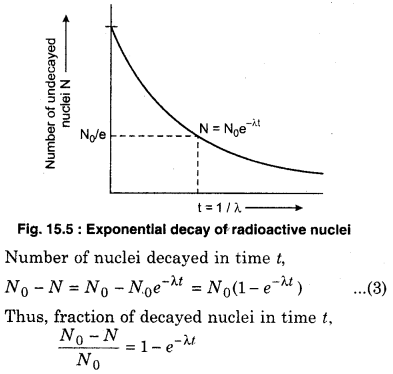
Where N0 is the number of active nuclei at time t = 0. From equation (2), it is apparent that the number of nuclei to be decayed decreases exponentially. It is shown in figure 15.5.
Question 7.
Give the definition of half life and mean life of a radioactive element and establish the relation between them.
Answer:
Half Life
We know that radioactive elements always disintegrated and as the time passes the number of united nuclei decreases. “Half life of a radioactive sample is the time to reduce the nuclei to half of its initial value.” Half life is denoted by T. Its value for an element is constant and varies for different elements. Half life does not depend on the quantity of substance. It cannot be replaced by physical and chemical effects.
The half life of some elements are given below :
| S. No. | Symbol | Half-life |
| 1. | Uranium (92U238) | 4.5 × 109 year |
| 2. | Thorium (90Th 230) | 8 × 104 year |
| 3. | Radium (88Ra 236) | 1620 year |
| 4. | Bismuth (83Bi218) | 3 min |
If a radioactive element has a half life of T then after T times it will have 50% of its initial value, after 2T times 25% and after 3T times 12.5%, after 4T time 6.25% remaining.
If the graph plotted between the number of nuclei and time, then the graph is shown in a figure 15.6.
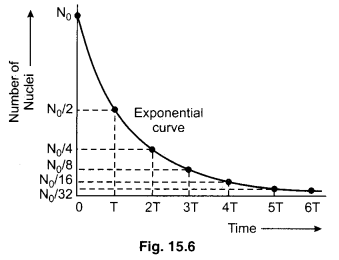
Let the number of nuclei in beginning is N0 that is N = 0 at t = 0. So remaining nuclei after a half life (t = T),
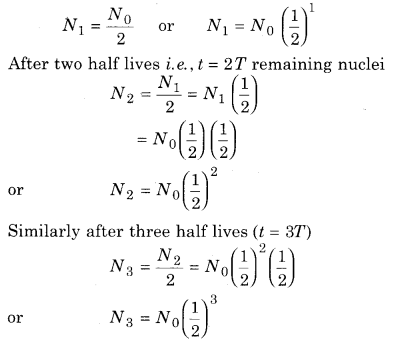
Similarly after three half lives (t = nT), remaining nuclear
Nn = N0 \(\left(\frac{1}{2}\right)^{n}\) ……………… (1)
∵ t = nT
Using n = \(\frac{t}{T}\), the value of n can be determined
Relation between Half-life and Decay constant
If the number of neclei in the beginning is N0 then the number of remaining nuclei after time t
N = N0e-λt
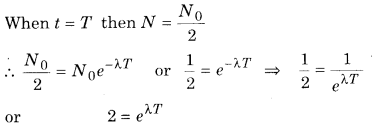
Taking log on both sides
loge = logeeλt = λt loge e = λt
or λt = loge 2
or T = \(\frac{\log _{e} 2}{\lambda}\) …………… (2)
With the help of this equation we can find out the value of λ it value of T is known or vice-versa,
∵ loge 2 = 0.6932
∴ T = \(\frac{0.6932}{\lambda}\) …………. (3)
or λ = \(\frac{0.6932}{T}\) …………….. (4)
Question 8.
What is α-decay? What type of energy spectrum do α-particles have?
Answer:
α-particle is the nucleus of helium which is double ionized particle.
ZXA → Z – 2YA – 4 + 2He4
Example : 92U238 → 90Th234 + 2He4
It is clear that the emission of α-particle reduces the atomic number of the original nucleus from two and the mass number is 4 that means the atomic number of the product nucleus decreases by 2 and the mass number is four, it is called α-decay.
Exposure from the nucleus of α-particles can be interpreted on the basis of quantum mechanics rather than coherent principles. According to that α-particle occurs only in the nucleus and by the tunnel effect it comes out of then nucleus. The spectrum of energy of α-particles is discrete and linear discrete energy spectrum demonstrates that nuclear power level is available in the nucleus as well as nuclear.
Question 9.
What does it mean when we say that β-ray spectrum is a continuous energy spectrum?
Answer:
A process in which a nucleus spontaneously emits an electron or a positron is called β-decay. The atomic number (Z) of daughter nucleus increases by unity, but the mass number (A) remains unchanged. Symbolically, (β– decay is represented as :
\(\underset{Z}{A} X \longrightarrow_{Z+1}^{A} Y+_{-1}^{0} e+\overline{v}\)
An example of β– decay is
\(\underset { 90 }{ ^{ 234 } } Th\longrightarrow _{ 91 }^{ 234 }Pa+_{ -1 }^{ 0 }e+\overline { v } \)
Here, we can see that charge and the number of nucleons are conserved in this type of decay. Antineutrino (\(\overline{\mathrm{V}}\)) is a neutral particle whose rest mass is very small. Total charge before reaction is +90e and charge after reaction – 91 e + (-e) + 0 = 90e. As electrons and anti-neutrino both are not nucleons. Thus, the number of nucleons remains the same, 234.
It is surprising that nucleus emits electrons (positrons) and neutrino because it has only neutrons and protons. In the previous chapter we have seen that an atom emits photons, however, we never say that photons are present in an atom. We say that photons are formed in the emission process. Similarly, in the β-decay, electrons (positrons) and antineutrino (neutrino) are formed. They are formed during the emission process.
We can also write.
n → p + e– + \(\overline{\mathrm{V}}\)
Here, we can see that nucleons do not vary in this process.
We can calculate disintegration energy in β– decay. Let the nuclear masses of X and Y be mx and my and mass of electron is me, then mass lost
Δm = mx – [my × me] ………….. (2)
Where antineutrino is considered to be massless. Adding and subtracting Zme on the right side of equation (2), we get
Δm = (mx + Zme) – [my +(Z + 1 )me]
or,Δm = Mx – My ……………….. (3)
Where, Mx and My the atomic masses of X and Y respectively, here, disintegration energy,
Q = Δmc2 = (Mx – My)c2 ………….. (4)
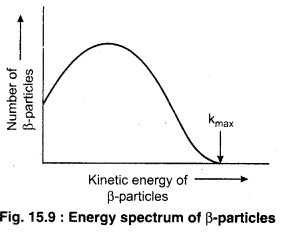
The emitted β-rays have a range of energy varying from zero to a maximum value. The distribution of kinetic energies of β-rays during β-decay is shown in figure 15.9.
In positive β(β+) decay, a positron and a neutrino are emitted from the nucleus. The symbolic representation of such a decay is
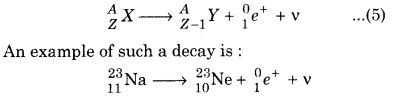
Here mass number A is unchanged where as atomic number reduces by unity. Like antineutrino, neutrino v is chargeless and almost massless. Neutrino and positrons are also formed during this,
p → n + e+ + v ………….. (6)
In β+ decay, like β– decay, there is conservation of number of nucleons and charge. The disintegration energy for β+ decay,
Q = (Mx – My – 2me)c2 ………… (15.31)
In some P decay, the parent nucleus accepts an electron and proton combines with it to form a neutron and emits a neutrino. Symbolic representation for such a decay is :
From β decay, we get to know that neutron and proton are not fundamental particles. It is note worthy that the process n → p + e– + v is possible both in the cases : inside and outside the nucleus, i.e., an isolated neutron can decay to form a proton. However the process, p → n + e+ + v is not possible outside the nucleus. As the mass of neutron is more than that of proton. Thus, it is not possible to decay an isolated proton into a neutron.
Question 10.
For explaining β-decay, which conservation law in the neutrino hypothesis is useful?
Answer:
Neutrino Hypothesis
Before 1930, it can considered that β-decay produces two particle-recoiling nucleus and electron (or positron), i. e.,
However, β-decay produced a wide spectrum of possible and smaller energies for electron.
A Swiss physicist Pauli postulated the existence of an electrically neutral, low mass particle that would be emitted along with the β-particle, for the validation of the law of conservation of energy. This particle was the neutrino. Later, Enrico Fermi proposed the theory of β-decay based on the hypothesis that an electron-neutrino pair is spontaneously produced by a nucleus in the same manner as the photons are spontaneously emitted by excited atoms.
This theory was the precursor of the theory of weak interaction. The neutrino remained a hypothetical particle until in 1956, evidence for its existence was broughts by Reines and Cowan. Thus, neutrino (invented by Pauli) played a vital role in the fundamentals of particle physics. It possess no electric charge and are affected solely by weak interactions.
Question 11.
Write any two properties of nuclear forces.
Answer:
Properties of nuclear forces.
(i) Nuclear forces are independent on electric charges. For a given system, the force between learn about charge distribution in the nucleus. Similarly, we get to know about the nuclear force (and not the nuclear charge) from the neutron-nucleus scattering experiment, from which we know about the mass distribution in the nucleus.
(ii) The range of nuclear forces is very small, of the order of nuclear size (of few femtometre), but from such type of scattering experiments, we inside this range, the nuclear force is much stronger than the electric force (50 ~ 60 times). Outside this range, nuclear forces are not two protons and two neutrons and that between 1 neutron and 1 proton is almost equal in magnitude. Electrons are not affected by the nuclear force. That is why in the scattering experiments by high energy electrons, electrons are scattered by the nuclear charge only and effective.
Question 12.
What is meant by binding energy per nucleon? How is it related with the stability of nucleus?
Answer:
According to Einstein, this mass (Δm) turns into energy. This energy is called binding energy. This energy binds all nucleons of the nucleus in the form of nucleus. Δm means that when the protons and neutrons together from the nucleus then Am mass disappears and its equivalent energy is released. By this energy the protons and neutrons are bound to the nucleus. It is obvious that breaking the proton and neutron of the nucleus will require the same outer energy. Thus it becomes clear that when the protons and neutrons from the nucleus then the some energy is released, which is called the binding energy. It is also clear from this fact that if the same energy is given to the nucleus then all nucleons will be free. Thus binding energy can also be defined as “The binding energy is the amount of energy that release all of the nucleons when giving it to the nucleus.” So, the binding energy of nucleus.
ΔE = Δmc2
= [{Zmp + (A – Z)mn} – m]uc2 …………. (2)
If the nucleon’s number divided in binding energy of nucleus then we get the binding energy per nucleon. Binding energy demonstrates the stability of the atom.
∴ Binding energy per nucleon = \(\frac{\Delta E}{A}\)
Question 13.
Define nuclear fission.
Answer:
Nuclear Fission
In 1932, after few years from the discovery of neutron, Fermi studied nuclear reactions by bombarding nuclei with other nuclear particles such as proton, neutron, α-particle, etc. According to Fermi, neutrons do not experience any Coulomb repulsive force on reaching the surface of a nucleus due to being neutral in nature. Thus, slow moving neutrons interact with the nucleons after entering the nucleus. Thermal neutrons are those neutrons which are in thermal equilibrium with the matter at room temperature. At 300 K, the kinetic energy of such neutrons is
Kav = \(\frac{3}{2}\)KT = \(\frac{3}{2}\)(8.62 × 10-5 eV / K) (300 K)
= 0.04 eV
These neutrons are effective and convenient for nuclear reactions.
In 1939, the German Scientists Otto Holen and Fritz Strassman bombared Uranium with thermal neutrons. In this process a new radioactive element was formed whose chemical properties were similar to that of Barium. Later it was found that it was not a new element and Barium (Z = 56) itself. Otto and Strassman found it difficult to understand- how Barium is formed when Uranium (Z = 92) is bombarded with neutrons?
The solution to this problem was presented by Physicists Lise Meittner and Otto Frisch. They showed that Uranium absorbs the thermal neutrons to break into two intermediate nuclei, and one of which could be Barium. This process was named nuclear fission. In nuclear fission, neutrons are also released with the product nuclei and the release of energy. It is to be noted that product nuclei are not unique as shown in the fission reactions of 235U.
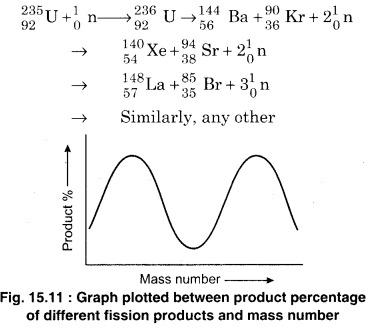
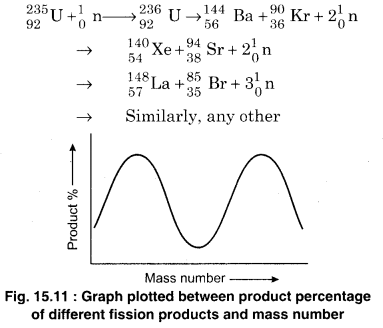
The two fission products of intermediate masses have different mass numbers. Generally, they are also radioactive. Such products, generally, undergo β– decay and it forms a chain till a stable product is obtained. A special example is as follows :
![]()
On an average, ~2.5 neutrons are obtained in a fission which are called fast neutrons. Energy of each of them is ~2MeV. Further fission of \(_{ 92 }^{ 235 }{ U }\) is not possible with these neutrons. Fig. 15.11 shows the graph between percentage of different fission product nuclei produced and the mass number. Different nuclides (above 100) which represents more than 20 different elements are shown. For most of the fission products, the mass number is from 90 to 100 and from 135 to 145.
The free energy Q for nuclear fission is more than that in chemical processes. To get its value, we use the binding energy per nucleon curve. In this curve, we see that binding energy per nucleon Em for heavy nuclei is around 7.6 MeV where as for intermediate nuclei, it is ~ 8.5 MeV. Consider a nucleus of mass number, A = 120 and the nucleus A = 120 splits into two nuclei then, total binding energy for A = 120 nucleus = ΔEbi
ΔEbi = ΔEbni A
ΔEbi = (7.6) × 240MeV
and total energy of 2 (A = 120) nuclei,
ΔEbf = 2(ΔEbnf )A/2 = 2(8.5) × 120
= (8.5) × 240 MeV
Thus, free energy in this process,
Q = ΔEbf – ΔEbi
= (8.5 – 7.6) × 240 = 216 MeV
Thus, fission energy Q is the order of ~ 200 MeV. This integration energy (Q) first appears as the kinetic energy of the neutrons and fragments. It finally is transferred to the surrounding.
Question 14.
What is meant by critical mass in nuclear chain reaction?
Answer:
Whether the mass of a fissionable material can sustain a chain reaction or not, depends on it multiplication factor. This in turn depends on the size of the material. The size of the fissionable material for which the multiplication factor k = 1 is called critical size and its mass is called critical mass. The chain reaction in this case remains steady or sustained.
Question 15.
Heavy water is a suitable moderator in a nuclear reactor, why?
Answer:
Moderator like heavy water contains a large number of protons. When fast neutrons are passed through it, they make elastic collisions with its protons, which have much smaller velocity. After few interactions, the velocities of neutrons get interchanged with those of protons. The final velocities of the neutrons are equal to the random velocities of the molecules of the moderator. The neutrons so obtained are called thermal neutrons.
RBSE Class 12 Physics Chapter 15 Long Answer Type Questions
Question 1.
Describe nuclear forces explaining the structure of nucleus.
Answer:
Nuclear Force
We have read that the size of nucleus is 10-15 m and in the nucleus the positive protons and neutral neutrons are present. There should be so much electrical repulsion between the protons of such a short distance that the nucleus cannot be stable, but the nucleus is still stable. It means that there is another force other than the gravitational and electric force, which keeps the nucleons in such a small place. This force is called nuclear force.
It could not be found yet, on changing the distance, the nuclear force changes according to which rule, but it is very certain that the rate of change of the nuclear forces on changing the distance is much higher than the gravitational force and the electrical force otherwise the nucleus is not stable. Gravitational force, Coulomb’s force and the nuclear force have the following ratio :
Fg : Fe : Fn = 1 : 1036 : 1038
The following facts have been found in about the nuclear force :
(i) Nuclear forces are independent on electric charges. For a given system, the force between learn about charge distribution in the nucleus. Similarly, we get to know about the nuclear force (and not the nuclear charge) from the neutron-nucleus scattering experiment, from
which we know about the mass distribution in the nucleus.
(ii) The range of nuclear forces is very small, of the order of nuclear size (of few femtometres), but from such type of scattering experiments, we inside this range, the nuclear force is much stronger than the electric force (50 ~ 60 times). Outside this range, nuclear forces are not two protons and two neutrons and that between 1 neutron and 1 proton is almost equal in magnitude. Electrons are not affected by the nuclear force. That is why in the scattering experiments by high energy electrons, electrons are scattered by the nuclear charge only and effective.
(iii) Nuclear forces are not central forces in nature. For the pairing of two nucleons, they do not depend upon the distance between them but also on the direction of their spin.
(iv) The density of nuclear matter is almost constant and the nuclear binding energy in the middle mass range is almost constant. This shows that each nucleon in a nucleus does not interact with the other nucleons present but interact with only some of them. (Think about a nucleon in a nucleus of mass number A. If it interacts with all other nucleons, then we will get interactions to be A (A – 1) / 2, then the total binding energy is proportional to A (A -1) and for A > >1, it is proportional to A2. This would mean the binding energy per nucleon is proportional to A i. e., it is not constant). This property of nuclear force is called “saturation nf nuclear force.” This is different from electric force. Each proton interacts with all other protons (and number of \(\frac{Z(Z-1)}{2} \sim Z^{2}\))
(v) Nuclear forces are dependent or spin or angular momentum of nucleons. Force between nucleons having parallel spins is greater than the force between nucleons having antiparallel spins.
(vi) Nuclear forces are due to exchange of π mesons between the nucleon,
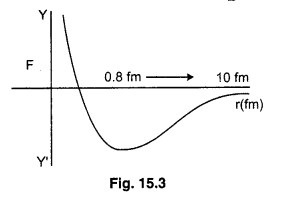
(vii) When the distance between nucleons becomes less than 0.8 fermi, the nuclear forces become strongly repulsive. The graph ploted between force and distance are given below :
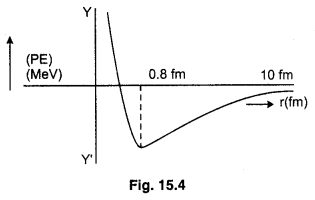
The potential energy is minimum at distance r0 = 0.8 fm. At this distance, force between nucleons is zero. For distance larger than 0.8 fm. negative potential energy goes on decreasing. The nuclear forces are attractive. For distance less than 0.8 fm. The negative potential energy decreases to zero and then becomes positive. The graph between potential energy and distance is given below :
(viii)Nuclear forces are generally attractive but repulsion is observed when the distance between the nucleons is found to be less than the order of 1 fm
Nuclear Structure
After the discovery of neutron in 1932 by James Chadwick, the scientist Heisenberg told that the nucleus is compared with protons and neutrons.
- The number of protons present in the nucleus is called atomic number (Z).
- The sum of the number of protons ad neutrons present in the nucleus in called mass number (A).
- The proton has a positive charge equal to the electronic charge, while the neutron is the neutral particle.
- The mass of the neutrons and protons present in the nucleus is almost identical. The mass of neutron is more than 0.5% of the mass of proton. Mass of Neutron mn = 1.67626231 × 10-27 kg
Mass of Proton mp – 1.6749286 × 10-27 kg - Electron is the original particle of nature, but neutrons and protons are not pure particles because they are made from other particles, they are known as Quarks.
- Nuclear number and mass number can be detected by the symbol of nuclear species or nuclides. According to the sign convention for nuclear species \(_{Z}^{A} X\) or AXZ or ZXA are used.
Where,
X: Chemical symbol of an element.
Z : Atomic number of a element which is equal to the number of proton present in nucleus.
A: Mass number of nuclear species which is also to the total number of nucleons.
N.: Number of neutrons in nucleus is given by the relation A – Z. - Neutron and proton present in the nucleus experienced the same nuclear force so the combined form of these are called nucleons.
Question 2.
Explain mass defect and binding energy. Explain the main results obtained from the graph between binding energy per nucleon and mass number.
Answer:
Mass Defect and Nuclear Binding Energy
We have read that the entire mass of the atom and positive charge is concentrated in the nucleus and nucleus is composed by the protons and neutrons. We also know how much protons and neutrons occur in the nucleus therefore, the expected mass of a nucleus can be determined by the calculation. The actual mass of nucleus can also be determined by mass spectrograph. It is found that the actual mass of any nucleus is always less than the expected mass obtained by its nucleons calculation. This difference of mass is called mass defect. Similarly,
Mass defect = Mass of nucleus obtained by the calculation — Actual mass of nucleus
or ∆m = mc – ma
Where calculated mass is represented by mc and actual mass is represented by ma.
∆m = [Mass of protons + mass of neutrons] – Actual mass of nucleus
or ∆m = [Zmp +(A – Z) mn] – m …………. (1)
Where Z is atomic number, A mass number, mp mass of proton, mn mass of neutron and m is the actual mass.
According to Einstein, this mass (∆m) turns into energy. This energy is called binding energy. This energy binds all nucleons of the nucleus in the form of nucleus. Am means that when the protons and neutrons together from the nucleus then Am mass disappears and its equivalent energy is released. By this energy the protons and neutrons are bound to the nucleus. It is obvious that breaking the proton and neutron of the nucleus will require the same outer energy. Thus it becomes clear that when the protons and neutrons from the nucleus then the some energy is released, which is called the binding energy. It is also clear from this fact that if the same energy is given to the nucleus then all nucleons will be free. Thus binding energy can also be defined as “The binding energy is the amount of energy that release all of the nucleons when giving it to the nucleus.” So, the binding energy of nucleus.
∆E = ∆mc2
= [{Zmp + (A – Z)mn} – m]uc2 ………….. (2)
If the nucleon’s number divided in binding energy of nucleus then we get the binding energy per nucleon. Binding energy demonstrates the stability of the atom.
∴ Binding energy per nucleon = \(\frac{\Delta E}{A}\)
Binding Energy per Nucleon
The ratio of the nuclear binding energy ∆Eb to its mass number A is called binding energy per nucleon. It is represented by ∆Ebn i.e.,
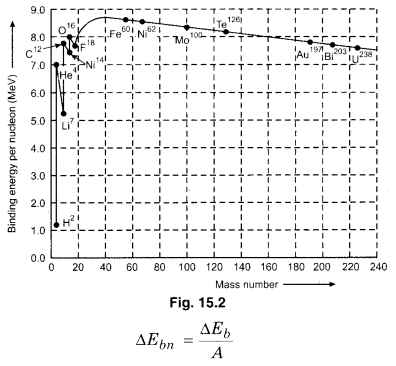
More the value of binding energy per nucleon for a nucleus. It shows that the stability of atom. If we plot a curve between binding energy per nucleon and the mass number A for the nuclei of different elements, we obtain a graph as shown in figure 15.2. From the study of this curve, we obtain the following results :
(i) The value of ∆Ebn first icnreases and attains the maximum value and then decreases slowly. For those nuclei, for which the value of number of nucleons, A is a multiple of 4, i.e.,A = 4, 8, 12, 16 the value of ∆Ebn is much more than that compared to the close nuclei. Thus, these are comparatively more stable. This tells that just like the shell structures in electrons, nucleons in the nucleus too have the same. For the inert gases, which have completely filled outermost shell, the atoms are comparatively more stable, 50 are the nuclei with the multiple of 4 have completely filled nuclear shells and hence are more stable. You will learn more about this in higher classes.
(ii) The nuclei of the mass number A ~ 50 to A ~ 80 are highly stable. The average ∆Ebn ~ 8.7 MeV per nucleon. For both A< 50 and A > 80, ∆Ebn decreases. The binding energy per nucleon near mass number A ~ 60, Ebn is maximum. Thus, the near by elements Fe, Ni, CO, etc. are highly stable. The binding energy per nucleon for 62Ni is maximum (8.8 MeV). This is most stable nucleus in nature. For \(_{ 26 }^{ 60 }{ Fe }\), it is a bit less than 8.8 MeV. That is why, molten, nickel and iron are highly found in the earth core.
(iii) For the mass numbers (30 < A < 170), Ebn is constant at about 8 MeV. In this range, ∆Ebn is not dependent on A
(iv) As mentioned above, the nucleus of medium mass number are more stable than those of high mass numbers. Thus, when a heavy nucleus breaks into two nuclei of medium nuclei, then the binding energy increases and rest mass energy decreases. Thus, energy is released in this process which appears in the form of kinetic energy of the obtained medium nuclei shows the possibility to release energy through the nuclear fission about which we will study later in this chapter.
(v) Similarly, we can imagine that two nuclei (A << 10) combines to form a heavy nucleus. For such type of light nuclei, the resultant medium nucleus has comparatively lesser binding energy per nucleon. Thus, here also the binding energy increases and rest mass enegy decreases. Thus, here also energy is released. We will discuss about this process of nuclear fusion later. Some other important results which we do not see from fig.
15.2. are :
1. For even A and even Z, the nuclei are generally stable and found in large amount.
2. In the general form, the nuclei of odd A and odd Z are unstable.
3. Exceptions are 2H, 6Li, 10B, 14N which are not stable while the nuclei of odd Z and even A are unstable.
Question 3.
Write the law of radioactive decay. Give the relation for half life and mean life using the law of exponential decay.
Answer:
Radioactivity
In the beginning of this chapter, it was mentioned that Henry Becquerel discovered radioactivity in 1896. Heavy elements like uranium, thorium, etc. particles or radiations on their own and undergoes a decay. In this process of decay, new atoms (elements) are formed that themselves exhibit radioactivity and this process continues till a stable element is formed.
The discovery of radioactivity by Henry Becquerel was purely accidental. He observed that when uranium salt-crystals or uranium-potassium sulphate were illuminated with visible light, they emitted some invisible radiations that blackened the photograhic plate placed near it which was covered by a light resistant cover. After this, scientists Marie Curie and Pierre Curie discovered the new elements radium and polonium from the pitch blend are which were more radioactive than uranium. Through the experiments, Rutherford showed that radioactive radiations are of three type which he called alpha, beta and gamma rays. The experiments performed later showed that-α (alpha) rays are helium nuclei, β (beta) rays are electrons or positrons and γ (gamma) rays are photons of high energy. Experiments also showed that the radioactivity is a result of decay of unstable nuclei. This way, we can say that radioactivity is a nuclear phenomenon. Some important facts related to radioactivity are as follows :
(i) External conditions like pressure temperature and state of radioactive substance (solid, liquid or gas) has no effect on radioactivity. Radioactivity is also unaffected by the chemical reactions or chemical combinations (like uranium or any of its compounds is radioactive). As there is contribution of only the outermost electrons in a chemical combination. Thus, the electronic configuration of atoms is not related to the radioactivity. Also, α-ravs, β-particles of very high energy or emission of γ-ray photons is not possible from the emission of outer part of atom. Thus, radioactivity is purely a nuclear phenomenon.
(ii) In the radioactive decay of a nuclei, with the laws of conservation of mass, energy, charge linear momentum, angular momentum and the conservation of number of nucleons should be followed.
(iii) A nucleus say X is unstable for the decay of α and β if its mass is more than the sum of its product constituents.
(iv) In the process of radioactive decay, emission energy per atom is the order of few MeV where as it of the order of few eV for chemical reactions.
Half Life
We know that radioactive elements always disintegrated and as the time passes the number of united nuclei decreases. “Half life of a radioactive sample is the time to reduce the nuclei to half of its initial value.” Half life is denoted by T. Its value for an element is constant and varies for different elements. Half life does not depend on the quantity of substance. It cannot be replaced by physical and chemical effects.
The half life of some elements are given below :
| S. No. | Symbol | Half-life |
| 1. | Uranium (92U238) | 4.5 × 109 year |
| 2. | Thorium (90Th230) | 8 × 104 year |
| 3. | Radium (88Ra236) | 1620 year |
| 4. | Bismuth (83Bi218) | 3 min |
If a radioactive element has a half life of T then after T times it will have 50% of its initial value, after 2T times 25% and after 3T times 12.5%, after 4T time 6.25% remaining.
If the graph plotted between the number of nuclei and time, then the graph is shown in a figure 15.6.

Let the number of nuclei in beginning is N0 that is N = 0 at t = 0. So remaining nuclei after a half life (t = T),
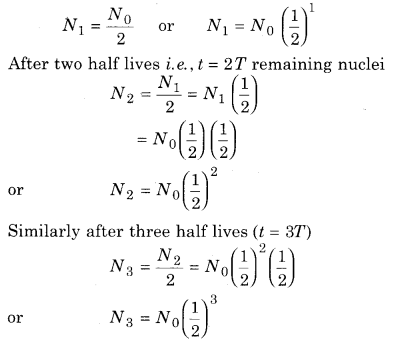
Similarly after three half lives (t = nT), remaining nuclear
Nn = N0 \(\left(\frac{1}{2}\right)^{n}\) ……………… (1)
∵ t = nT
Using n = \(\frac{t}{T}\), the value of n can be determined
Relation between Half-life and Decay constant
If the number of neclei in the beginning is N0 then the number of remaining nuclei after time t
N = N0e-λt
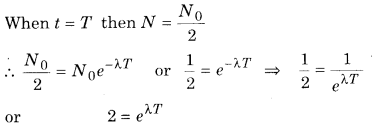
Taking log on both sides
loge = logeeλt = λt loge e = λt
or λt = loge 2
or T = \(\frac{\log _{e} 2}{\lambda}\) …………… (2)
With the help of this equation we can find out the value of λ it value of T is known or vice-versa,
∵ loge 2 = 0.6932
∴ T = \(\frac{0.6932}{\lambda}\) …………. (3)
or λ = \(\frac{0.6932}{T}\) …………….. (4)
Question 4.
What is nuclear fission? Why is fission not self-sustaining chain reaction? Explain what is done to obtain chain reaction?
Answer:
Nuclear Fission
In 1932, after few years from the discovery of neutron, Fermi studied nuclear reactions by bombarding nuclei with other nuclear particles such as proton, neutron, α-particle, etc. According to Fermi, neutrons do not experience any Coulomb repulsive force on reaching the surface of a nucleus due to being neutral in nature. Thus, slow moving neutrons interact with the nucleons after entering the nucleus. Thermal neutrons are those neutrons which are in thermal equilibrium with the matter at room temperature. At 300 K, the kinetic energy of such neutrons is
Kav = \(\frac{3}{2}\)KT = \(\frac{3}{2}\)(8.62 × 10-5 eV / K) (300 K)
= 0.04 eV
These neutrons are effective and convenient for nuclear reactions.
In 1939, the German Scientists Otto Holen and Fritz Strassman bombared Uranium with thermal neutrons. In this process a new radioactive element was formed whose chemical properties were similar to that of Barium. Later it was found that it was not a new element and Barium (Z = 56) itself. Otto and Strassman found it difficult to understand- how Barium is formed when Uranium (Z = 92) is bombarded with neutrons?
The solution to this problem was presented by Physicists Lise Meittner and Otto Frisch. They showed that Uranium absorbs the thermal neutrons to break into two intermediate nuclei, and one of which could be Barium. This process was named nuclear fission. In nuclear fission, neutrons are also released with the product nuclei and the release of energy. It is to be noted that product nuclei are not unique as shown in the fission reactions of 235U.
The two fission products of intermediate masses have different mass numbers. Generally, they are also radioactive. Such products, generally, undergo β– decay and it forms a chain till a stable product is obtained. A special example is as follows :
![]()
On an average, ~2.5 neutrons are obtained in a fission which are called fast neutrons. Energy of each of them is ~2MeV. Further fission of \(_{ 92 }^{ 235 }{ U }\) is not
possible with these neutrons. Fig. 15.11 shows the graph between percentage of different fission product nuclei produced and the mass number. Different nuclides (above 100) which represents more than 20 different elements are shown. For most of the fission products, the mass number is from 90 to 100 and from 135 to 145.
The free energy Q for nuclear fission is more than that in chemical processes. To get its value, we use the binding energy per nucleon curve. In this curve, we see that binding energy per nucleon Em for heavy nuclei is around 7.6 MeV where as for intermediate nuclei, it is ~ 8.5 MeV. Consider a nucleus of mass number, A = 120 and the nucleus A = 120 splits into two nuclei then, total binding energy for A = 120 nucleus = ΔEbi
ΔEbi = ΔEbni A
ΔEbi = (7.6) × 240MeV
and total energy of 2 (A = 120) nuclei,
ΔEbf = 2(ΔEbnf )A/2 = 2(8.5) × 120
= (8.5) × 240 MeV
Thus, free energy in this process,
Q = ΔEbf – ΔEbi
= (8.5 – 7.6) × 240 = 216 MeV
Thus, fission energy Q is the order of ~ 200 MeV. This integration energy (Q) first appears as the kinetic energy of the neutrons and fragments. It finally is transferred to the surrounding.
Explanation of nuclear fission by liquid drop model
Nuclear fission can be understood by the liquid drop model proposed by Bohr and Wheeler. Here, we will study the very simple form of this model in brief. In the form of a liquid drop, a nucleus can be considered to be a spherically charged liquid drop which is in equilibrium due to the balance between the internal attractive forces and Coulomb repulsive force. Fig. 15.12 shows the fission process for 235U nucleus. When a heavy nucleus like 235U absorbs a slow moving neutron, then the potential energy related to the nuclear nucleons transforms into the internal excitation energy of the nucleus. In this process, the excitation energy is around 6.5 MeV and the nucleus starts vibrating due to excitation energy (fig. 15.10). Due to these vibration, a structure like a double is formed is breaks into two fragments due to the repulsion between the two otherwise, the nucleus would emit γ-rays to come back to initial state. Calculations by Bohr and Wheeler based on quantum mechanics, shows that energy required to break the 235U nucleus, which is called critical energy is around 5.3 MeV, which is less than the excitation energy. Thus, nuclear fission is possible.
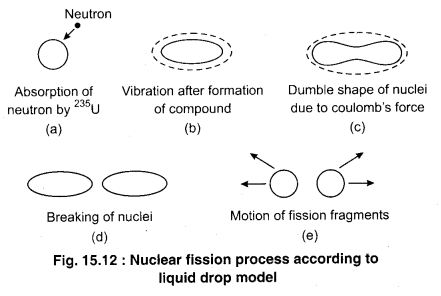
If the excitation energy of a nucleus on absorption of slow neutrons is less than the critical energy, then fission is not possible with such neutrons. For example, it is possible for 239U, but not for 238U and 244 Am. However, fission of these nuclei is possible with fast moving neutrons.
Question 5.
Explain the process of nuclear reactor with the help of schematic diagram.
Answer;
Nuclear Reactor
With the help of nuclear reactors, power is generated by changing the energy obtained from nuclear fission into electric energy. As we have already mentioned the controlled chain reactions are used in nuclear reactors. Here we will discuss nuclear reactor based on fission of 235U by thermal neutrons. Such type of reactor and equipments related to it are shown by a simple diagram in fig. 15.14. The core of the reactor contains Uranium as the nuclear fuel in suitably fabricated form. The core contains a moderator (water, heavy water (D20) or graphite) to slow down the moderator.
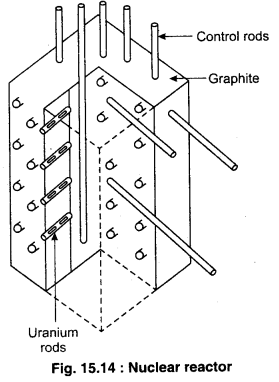
When fission takes place in a Uranium rod, then the fast neutrons produced enter the moderator from the rods where they are collided with the atoms of moderator. After few collisions, the energy of the neutrons decreases from 2 MeV to that of the range of thermal energy. The distance between the rods are so arranged that the neutrons come into the range of thermal energy before entering into a rod after emerging from rod. This way, the neutrons in the range of 1-100 eV have lesser possibility to absorb the 238U nuclei present in a rod. The geometry of core is such that the loss of neutrons is limited so that on an average, 1 out of 2.5 neutrons per fission is available in the next fission. In this case, multiplication factor, K – 1 and the chain reaction continues at a stable rate. If the rate of loss of neutrons decreases, then K > 1, and there is a possibility of explosion in the reactor and to solve this problem, control rods which are of Cadmium are used. Cadmium are good absorbers of neutrons. Initially, it is kept at K > 1. We can get the state of K = 1 when required, Cadmium rods are inserted completely to get the state’of K < 1 and the nuclear reactor can be shut down. This is done for the safety reasons and proper treatment of reactors.
Due to the energy obtained from the nuclear high pressure fission in reactor, water or molten Sodium at high pressure is passed in the region of core to remove heat, which acts as a coolant. The heat taken from reactor by the coolant is used to convert water into steam. This steam is used to drive the turbine and to produce electricity.
Thick concrete walls are made around reactor so that the radioactive radiations from the fission products do not enter the atmosphere. This is for the safety of people working in and near the nuclear power production centres.
The other uses than the power production of nuclear reactors is to produce radioactive isotopes, research for neutrons, etc. Although nuclear reactors contribute towards electric energy production, but the disposal of its toxic radioactive wastes is a big problem for which it is very important to follow .the international rules. Also, its operation in done with strict management. Carelessness in safety management can be lethal for years. In 1986 in Russia (Ukraine in present), such incident happened in Chernobyl nuclear plant, whose result can till be seen in the local people. Indian Atomic Energy Programme is in accordance with the international systems in view of safety.
Question 6.
Explain β-decay. Describe the neutrino hypothesis is β decay.
Answer:
A process in which a nucleus spontaneously emits an electron or a positron is called (3-decay. The atomic number (Z) of daughter nucleus increases by unity, but the mass number (A) remains unchanged. Symbolically, (β– decay is represented as :
\(\underset { 90 }{ ^{ 234 } } Th\longrightarrow _{ 91 }^{ 234 }Pa+_{ -1 }^{ 0 }e+\overline { v } \)
An example of β– decay is
\(\underset{90}{234} Th \longrightarrow_{91}^{234} Pa+_{-1}^{0} e+\overline{v}\)
Here, we can see that charge and the number of nucleons are conserved in this type of decay. Antineutrino (\(\overline{\mathrm{V}}\)) is a neutral particle whose rest mass is very small. Total charge before reaction is +90e and charge after reaction – 91 e + (-e) + 0 = 90e. As electrons and anti-neutrino both are not nucleons. Thus, the number of nucleons remains the same, 234.
It is surprising that nucleus emits electrons (positrons) and neutrino because it has only neutrons and protons. In the previous chapter we have seen that an atom emits photons, however, we never say that photons are present in an atom. We say that photons are formed in the emission process. Similarly, in the β-decay, electrons (positrons) and antineutrino (neutrino) are formed. They are formed during the emission process.
We can also write.
n → p + e– + \(\overline{\mathrm{V}}\)
Here, we can see that nucleons do not vary in this process.
We can calculate disintegration energy in β– decay. Let the nuclear masses of X and Y be mx and my and mass of electron is me, then mass lost
Δm = mx – [my × me] ………….. (2)
Where antineutrino is considered to be massless. Adding and subtracting Zme on the right side of equation (2), we get
Δm = (mx + Zme) – [my +(Z + 1 )me]
or,Δm = Mx – My ……………….. (3)

Where, Mx and My the atomic masses of X and Y respectively, here, disintegration energy,
Q = Δmc2 = (Mx – My)c2 ………….. (4)
The emitted β-rays have a range of energy varying from zero to a maximum value. The distribution of kinetic energies of β-rays during β-decay is shown in figure 15.9.
In positive β(β+) decay, a positron and a neutrino are emitted from the nucleus. The symbolic representation of such a decay is
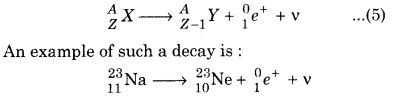
Here mass number A is unchanged where as atomic number reduces by unity. Like antineutrino, neutrino v is chargeless and almost massless. Neutrino and positrons are also formed during this,
p → n + e+ + v ………….. (6)
In β+ decay, like β– decay, there is conservation of number of nucleons and charge. The disintegration energy for β+ decay,
Q = (Mx – My – 2me)c2 ………… (15.31)
In some P decay, the parent nucleus accepts an electron and proton combines with it to form a neutron and emits a neutrino. Symbolic representation for such a decay is :
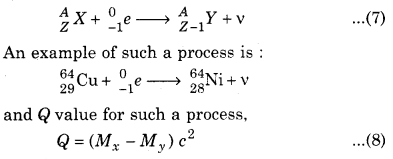
From β decay, we get to know that neutron and proton are not fundamental particles. It is note worthy that the process n → p + e– + v is possible both in the cases : inside and outside the nucleus, i.e., an isolated neutron can decay to form a proton. However the process, p → n + e+ + v is not possible outside the nucleus. As the mass of neutron is more than that of proton. Thus, it is not possible to decay an isolated proton into a neutron.
Neutrino Hypothesis
Before 1930, it can considered that β-decay produces two particle-recoiling nucleus and electron (or positron), i. e.,
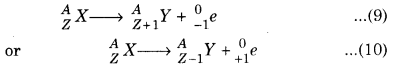
However, β-decay produced a wide spectrum of possible and smaller energies for electron.
A Swiss physicist Pauli postulated the existence of an electrically neutral, low mass particle that would be emitted along with the β-particle, for the validation of the law of conservation of energy. This particle was the neutrino. Later, Enrico Fermi proposed the theory of β-decay based on the hypothesis that an electron-neutrino pair is spontaneously produced by a nucleus in the same manner as the photons are spontaneously emitted by excited atoms.
This theory was the precursor of the theory of weak interaction. The neutrino remained a hypothetical particle until in 1956, evidence for its existence was brought by Reines and Cowan. Thus, neutrino (invented by Pauli) played a vital role in the fundamentals of particle physics. It possess no electric charge and are affected solely by weak interactions.
Question 7.
Describe α-decay in a radioactive nucleus. Explain that the energy spectrum of α-particles obtained from the decay is wide spectrum of group of energies.
Answer:
α-Decay
The nucleus which emits the α-particle, is called the parent nuclus. When an external nucleus emits α-particles the internal energy of the parent nucleus is very high and it is stable. For emission of additional energy, the parent nucleus emits an α-particle and the built nucleus is called the daughter nucleus. This daughter nucleus expresses the mass and velocity by md and vd respectively.
The equation of α-decay is written as follows :
ZXA → Z – 2YA – 4 + 2He4 + Q(energy)
In the equation the number of nucleons and charge are conserved.
92U238 → 90Th234 + 2He4
By the linear momentum conservation
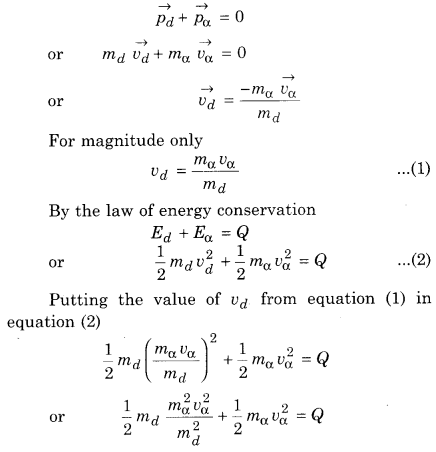
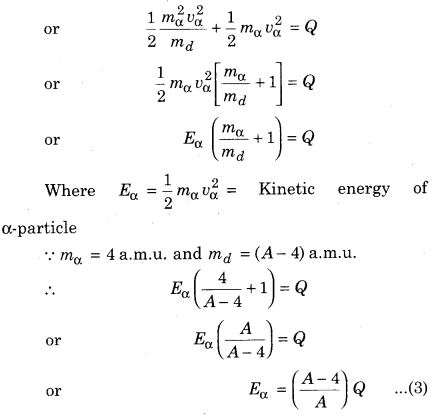
The daughter nucleus is very heavy compared to α-particle, so almost the entire disintegration energy appears as the kinetic energy of the α-particle.
For example, at the time of α-decay from the 94Po210 nucleus the kinetic energy of the α-particle is 5.3 MeV then by the equation (3),
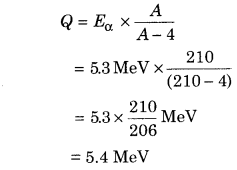
It is clear that disintegration energy almost appears in the form of kinetic energy of α-particle. If the potential barrier of nucleus is like the kinetic energy of α-particle then the α-particle may get rid of. It has been seen that the potential barrier of nucleus is 26 MeV.
According to quantum Mechanics, α-particles whose energy is 5.4 MeV does not exceed the potential barrier. But the energy of any α-particle emitted by the radioactive is not so much, so it can not escape from the nucleus. The problem of the escape of the nucleus of α-particle is solved by Gamaw. Condon and Gurney in 1928. According to this theory, α-particles are present in the nucleus before the emission of the nucleus.
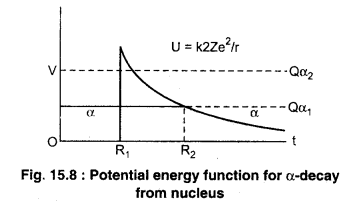
(i) When the α-particle occurs in the nucleus, the nuclear attraction force work on it (r < R1).
(ii) When the α-particle comes out of the nucleus then the unstable repulsive force acts between the α-particles in the nucleus (r < R2) Disintegration energy of α-decay is almost equal to the kinetic energy of α-particle.
Question 8.
How is proton-proton cycle completed in fusion. Why these thermonuclear reactions cannot be done is laboratories?
Answer:
Nuclear Fusion
When two light nuclei fuse together to form a large nucleus and energy is released, then the process is called nuclear fusion. The mass of the nucleus obtained from the fusion is less than the sum of the masses of the nuclei that fuse so, the loss in mass of the nucleus is transformed in the form of energy in accordance with the mass-energy equation. We have cleared this fact in the section of binding energy per nucleons.
Nuclear fusion is a complex process. For fusion, it in important to bring the nuclei to be fused so close that that these are in the range of nuclear forces, which is a difficult task. When nuclei are brought close, then the acting Coulomb repulsive force is strong. To overcome this Coulomb force, the kinetic energy of the nuclei should be in the range of 0.1 MeV to 1 MeV. These energies can be obtained from particle accelerators but in accelerators, the possibility of radiating is more for nucleus than fusion. Also, to collide a nucleus with another, the energy invested in accelerator is more as compared to the energy obtained from fusion. To obtain energy from fusion, the matter particles in the gaseous form are heated at high temperature so that the nuclei come so close that fusion occurs. This temperature is in the range of 109K and this type of fusion reaction is called thermonuclear fusion.
An example of fusion reactions is shown below in the form of fusion of deuterium nuclei :
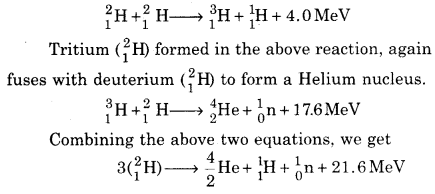
Thus three nuclei of deuterium combine to form a nucleus of Helium \(\left(\begin{array}{l}{4} \\ {2}\end{array} \mathrm{He}\right)\) nucleus and 21.6 MeV energy is released. Although the energy obtained from fusion (21.6 MeV) is quite less than the fission of a Uranium nucleus (200 MeV), but if we compare the fusion energy of deuterium per kilogram to fission energy of Uranium per kilogram, then it is very low. Also, no radioactive products are formed in fusion whereas the products in fission are radioactive. Thus, comparatively, fusion energy is a clean source.
RBSE Class 12 Physics Chapter 15 Numerical Questions
Question 1.
The radius of a nucleus of nucleon number 16 is 3 × 1015 m. What is the radius of a nucleus with nucleon number 128?
Solution:
Given, A1 = 16
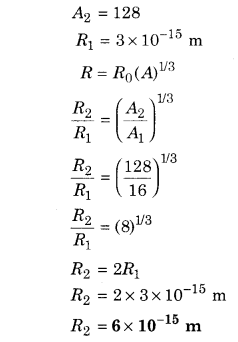
Question 2.
Calculate binding energy for \(_{ 26 }^{ 56 }{ Fe }\) nucleus [Given : mass of \(_{ 26 }^{ 56 }{ Fe }\) = 55.9349 u, mass of neutron = 1.00867 u, mass of proton = 1.00783 u and 1 u= 931MeV/c2] .
Solution:
Mas defect
Δm = [(30mn + 2mp) – M (26Fe56)]
Δm = (30 × 1.00867+ 26 × 1.00783)u – 55.9349u
Δm = (30 × 2601 + 26.20358) u – 55.9349 u
Δm = 56.46368 – 55.9349u
Δm = 0.52878u
EB = 0.52878 × 931 MeV
EB = 492.29 MeV ≈ 492 MeV
Question 3.
The half life of a radioactive isotope X is 3 s. Initially there are 8000 atoms of this isotope in the sample. Calculate (i) its decay constant (ii) time t in which 1000 atoms are active in the sample.
Solution:
T1/2 = 3 S, N0 = 8000
(i) We know that
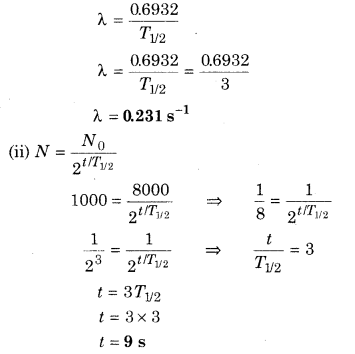
Question 4.
A radioactive nucleus decays as \(X \stackrel{\alpha}{\longrightarrow} X_{1} \stackrel{\beta^{-}}{\longrightarrow} X_{2} \stackrel{\alpha}{\longrightarrow} X_{3} \stackrel{\gamma}{\longrightarrow} X_{4}\)
If the mass number of X is 180 and atomic number is 72, then calculate the mass number and atomic number of X4.
Solution:
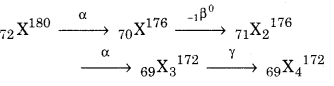
Atomic number of X4 = 69
Mass number = 172
Question 5.
In the fusion reaction,
\(_{1}^{2} \mathbf{H}+_{1}^{2} \mathbf{H} \longrightarrow_{2}^{3} \mathbf{H} \mathbf{e}+_{0}^{1} n\)
The masses of deuteron, helium and neutron are 2.015 u, 0.017 u and 1.009 u respectively. If 1 kg of deuteron completely undergoes fusion, then calculate free energy.
[1u = 9.31 MeV/c2 ]
Solution:
There are 6.02 × 1023 atoms in 1 mole (0.002 kg) of deuterium.
∴ Number of atoms in 1 kg of deuterium
= \(\frac{6.023 \times 10^{23}}{0.002}\)
= 3.01 × 1026
From above equation, for produced energy from fusion of deuterium
Δm = [2 × 2.015 u – (3.017 + 1.009) u]
Δm = [4.030u – 4.026u]
Δm = 0.004
EB = Δmc2
EB = 0.004 × 931 × \(\frac { { Me }{ V } }{ c^{ 2 } } c^{ 2 }\)
EB = 3.724 Me V
Fusion energy of 2 deuterium = 3.724 MeV
Fusion energy of 1 deuterium = \(\frac{3.724}{2}\) Me V = 1.862 Me V
Fusion energy of 1 kg deuterium
= (1.862 × 3.01 × 1026) MeV
= 5.6 × 1026 MeV
= 5.6 × 1026 × 106 × 1.6 × 10-19J
= 8.96 × 1013
= 9 × 1013 J
Question 6.
Calculate Q-value for the reaction
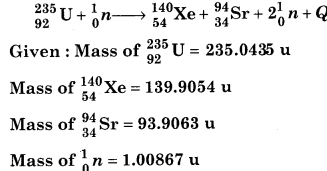
Solution:
Mass defect
Δm = [M (92U235) + M (0n1) – M (54Xe140) – M (38Sr94) – M (20n1)]
Δm = [(235.0435 + 1.00867) -(139.9054 + 93.9063) + 2 × 1.00867]
Δm = [(236.05217)-(235.82904)] u
Δm = 0.22313u
Q = Δmc2
Q = 0.22313 × 931\(\frac{\mathrm{MeV}}{c^{2}} c^{2}\) [∵ 1 u = 931\( \frac{\mathrm{MeV}}{c^{2}}\)]
Q = 207.73 MeV
Question 7.
Calculate the amount of 237Th for lmCi activity, whose half life is 19 years.
Solution:
1 millicurie = 10-3 curie = 10-3 × 3.7 × 1010 Disintegration/ sec
Activity of Thorium R = 3.7 × 107 disintegration/sec
R = λN
R = \(\frac{0.6932}{T_{1 / 2}} N\)
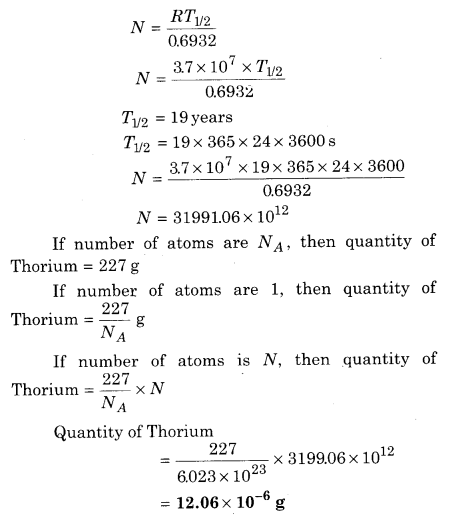
Question 8.
The activity for a sample of radioactive element found in an experiment is 6400 decays per minute. Repeating this experiment after 6 days, activity was found to be 400 decays per minute. Calculate half life of the given element.
Solution:
We know that

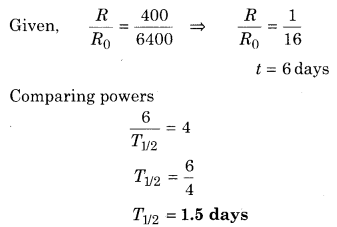
Question 9.
α-particle is emitted from a 88Ra226 nucleus. If the energy of a-particle is 4.662 MeV, then what is the total free energy in this decay?
Solution:
88Ra226
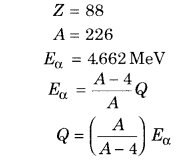
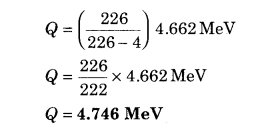
Question 10.
176X nucleus decomposes by β-decay to form 176Y. If the atomic masses of X and Y are 175.942694 u and 175.941426 u respectively. Then calculate the maximum energy of emitted β-particle.
Solution:
X176 → Y179 + -1β0
Mass defect Δm = [175.942694 – 175.941426] u
Δm = 0.001268 u
Maximum K.E. of β-particles
= Δmc2 × 1 u
= Δmc2 × \(\frac{931}{c^{2}}\) Me V
= Δm × 931 MeV
= 0.001268 × 931 MeV
= 1.18 Me V