RBSE Class 12 Chemistry Model Paper 1 English Medium are part of RBSE Class 12 Chemistry Board Model Papers. Here we have given RBSE Class 12 Chemistry Sample Paper 1 English Medium.
| Board | RBSE |
| Textbook | SIERT, Rajasthan |
| Class | Class 12 |
| Subject | Chemistry |
| Paper Set | Model Paper 1 |
| Category | RBSE Model Papers |
RBSE Class 12 Chemistry Sample Paper 1 English Medium
Time: 3¼ Hours
Maxim Marks: 80
General Instructions to the Examinees:
- Candidate must write first his/her Roll No. on the question paper compulsorily.
- All the questions are compulsory.
- Write the answer to each question in the given answer-book only,
- For questions having more than one part, the answer to those parts are to be written together in continuity.
- If there is any error/difference/contradiction in Hindi & English version of the question paper, the question of Hindi version should be treated valid.
-
Question Nos. Marks Per Questions 1 – 13 1 14 – 24 2 25 – 27 3 28 – 30 4 - Question Nos. 21, 27, 28, 29 and 30 have internal choices.
SECTION-A
Question 1.
Which type of semiconductor is formed when Arsenic is dopped with Germanium? [1]
Question 2.
Write detination of osmotic pressure. [1]
Question 3.
Draw a labeled diagram of dialysis method, for purification of colloidal solutions. [1]
![]()
Question 4.
Write electronic configuration of Chromium (Z = 24). [1]
Question 5.
Write name and symbol of one transurenic element. [1]
Question 6.
Write general oxidation state of Lanthanoids. [1]
Question 7.
Write IUPAC name of the following complex compound. [1]
K3[Fe(C2O4)3]
Question 8.
Write chemical equation of Wurtz reaction. [1]
Question 9.
Write full name of DDT. [1]
Question 10.
Write IUPAC name and chemical formula of Acetone. [1]
![]()
Question 11.
Write the formula to determine ‘weight average molecular weight’ of polymers. [1]
Question 12.
Write Fischer Projection Formula of erythrose sugar. [1]
Question 13.
Which chemical substance is found in musk, excreted by male musk deer? [1]
SECTION-B
Question 14.
(A) Draw a neat and labeled diagram of “blast furnace.” [1 + 1=2]
(B) What is the role of Silica in the copper metallurgy?
Question 15.
(A) Copper shows electrical conductance in solid as well as molten state whereas copper chloride shows electrical conductance only in molten state. Give reason. [1+1=2]
(B) Draw a diagram of “two dimension square closed packing”.
Question 16.
- Generally, solubility of gases in liquids is decreases as increasing temperature, Give. reason. [1 + 1=2]
- How many grams of NaCl is required to make 200ml aqueous solution of 5% (w/v) NaCl?
Question 17.
(A) Draw a labeled diagram of ‘Standard Hydrogen Electrode’. [1 + 1=2]
(B) Fuel Cells are better than other cells. Give any two reasons.
![]()
Question 18.
On the basis of valence bond theory explain the oxidation state, hybridisation, geometry and magnetic nature of metal in complex [CoF6]3-. [2]
Question 19.
(A) Arrange the following alkyl halides in ascending order of their reactivity towards SN1 reaction. [1 + 1=2]
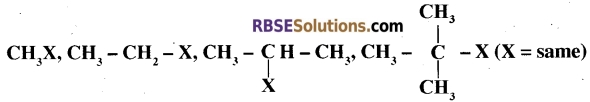
(B) Complete the following chemical reactions and write the products.
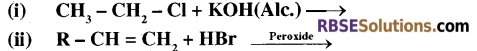
Question 20.
A solution of copper sulphate electrolysed for 20 minutes with a current of 1.5 Ampere. Calculate the mass of copper deposited at the cathode. (F = 96500 C) [2]
Question 21.
(A) Arrange the following compounds in the descending order of their reactivity towards nucleophilic substitution reaction. [1 + 1=2]
CH3CHO, HCHO, CH3COCH3
(B) Alkanoic acid have highest boiling points. Explain.
OR
(A) Arrange the following carboxylic acid in ascending order of their acidity. Benzoic acid, 4–methoxybenzoic acid, 4-nitrobenzoic acid.
(B) How will you distinguish between Aldehyde and Ketone by chemical test?
Question 22.
(A) Write the name of monomer unit of polymer used in non-stick surface coates utensils. [1 + 1=2]
(B) Give an example of each of Homopolymer and copolymer.
Question 23.
(A) Which conformer has higher energy in the Sawhorse projection formula of ethane and why? [1 + 1=2]
(B) Racemic mixture are optically inactive. Give reason.
![]()
Question 24.
(A) Aspirin should not be taken in empty stomach, why? [1 + 1=2]
(B) Write any two differences between dyes and pigments.
SECTION – C
Question 25.
Read the given paragraph and write answers of the following questions. [1 +1 +1=3]
When any solid substance is kept in contact with liquid or gas, then liquid or gas are more adsorbed on the surface of solid rather than bulk. The process is known as adsorption. It is different from absorption. Many gaseous reactions occur in the presence of solid catalyst. Catalyst is a chemical substance which change the rate of reaction without undergoing itself change. This phenomenon is known as catalysis.
(A) Write any two differences between Absorption and Adsorption.
(B) Write any chemical equation of heterogeneous catalysis.
(C) Write the name of Zeolite catalyst used to convert Alcohol to Petrol.
Question 26.
Read the given paragraph and write answers of the following questions. [2 + 1 = 3]
Protein is very essential for the growth, development and maintenance for living systems. Proteins are natural polymer of a-amino acids. A definition sequence of amino acids form a specific protein. Two or more than two amino acids bind to give peptide bond. Proteins are polypeptide, which loses is biological activity by physical or chemical changes.
(A) Explain essential and non- essential amino acid with example.
(B) Explain denaturation of protein.
Question 27.
(A). Complete the following equations and identify A and B. [2 + 1 = 3]
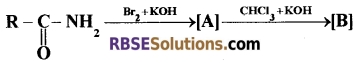
(B). Draw the resonating structures of Urea.
OR
(A). Complete the following equations and identify A and B.
![]()
(B). Draw the resonating structures of Aniline.
SECTION – D
Question 28.
(A). Write definition of order of reaction. [1 + 1 + 2 = 4]
(B). According to collision theory, write two main barriers for any chemical reaction.
(C). Show that, In a first order reaction, time required for completion of 99.9% is 10 times of half-life, (log 10 = 1)
OR
(A). Write definition of Molecularity of reaction.
(B). According to collision theory, write name of two factors which increasing the rate of reactions as temperature increase.
(C). Show that in a first order reaction, time required for completion of 75% twice of half-life of the reaction, (log 2 = 0.3010)
![]()
Question 29.
(A). Molecular formula of oxygen is O2 while sulphur is S8 why?
(B). Explain with chemical equation-what happens when slacked lime reacts with chlorine?
(C). Complete the following reaction.
C2H5OH + PCl5 →
(D). Draw Structure of XeO3 [ 1 + 1 + 1 + 1 = 4 ]
OR
(A). H2S is gas while H2O is liquid. Why?
(B). Explain with chemical equation-What happen when white phosphorous is heated at 473K and very high pressure?
(C). Complete the following reaction.
XeF6 + 2H2O →
(D). Draw structure of HClO4.
Question 30.
(A). Write the mechanism of dehydration of ethanol to form ethane.
(B). Give the name of Enzyme used to convert glucose into ethanol.
(C). Write chemical equation of phenol with chloroform and KOH. [2 + 1 + 1 = 4]
OR
(A). How to convert methanol into ethanol? Writhe chemical equations only.
(B). The mixture of alcohol and Ether, used in place of petrol, is known as?
(C). How will you obtain p-hydroxybenzaldehyde from phenol? Give chemical equation.
![]()
We hope the given RBSE Class 12 Chemistry Model Paper 1 English Medium will help you. If you have any query regarding RBSE Class 12 Chemistry Sample Paper 1 English Medium, drop a comment below and we will get back to you at the earliest.