RBSE Class 12 Chemistry Model Paper 2 English Medium are part of RBSE Class 12 Chemistry Board Model Papers. Here we have given RBSE Class 12 Chemistry Sample Paper 2 English Medium.
| Board | RBSE |
| Textbook | SIERT, Rajasthan |
| Class | Class 12 |
| Subject | Chemistry |
| Paper Set | Model Paper 2 |
| Category | RBSE Model Papers |
RBSE Class 12 Chemistry Sample Paper 2 English Medium
Time: 3¼ Hours
Maxim Marks: 80
General Instructions to the Examinees:
- Candidate must write first his/her Roll No. on the question paper compulsorily.
- All the questions are compulsory.
- Write the answer to each question in the given answer-book only,
- For questions having more than one part, the answer to those parts are to be written together in continuity.
- If there is any error/difference/contradiction in Hindi & English version of the question paper, the question of Hindi version should be treated valid.
-
Question Nos. Marks Per Questions 1 – 13 1 14 – 24 2 25 – 27 3 28 – 30 4 - Question Nos. 21, 27, 28, 29 and 30 have internal choices.
SECTION-A
Question 1.
Write names of any two interstitial voids. [1]
Question 2.
Write the names of any two factors which effect the solubility of gases. [1]
Question 3.
Draw a labeled diagram of Bredig’s arc method. [1]
![]()
Question 4.
Write the formula to determine magnetic moment. [1]
Question 5.
Write names of the elements which show maximum and minimum number of oxidation states in first transition series. [1]
Question 6.
Write the general electronic configuration of rare earth elements. [1]
Question 7.
Write names of any two ambident ligands. [1]
Question 8.
Write the formula of Freon-112. [1]
Question 9.
Write Riemer-Tiemann reaction. [1]
![]()
Question 10.
Write the chemical formula and IUPAC name of Chloral. [1]
Question 11.
Write the formula to determine ‘number average molecular weight’ of polymers. [1]
Question 12.
Write Fisher projection formula of mesotartric acid. [1]
Question 13.
Write any two properties of Dyes. [1]
SECTION-B
Question 14.
Draw a labeled diagram of Bessemer convertor. Write the chemical reaction of self reduction involved in it. [1 + 1=2]
Question 15.
Explain conductors, semiconductors, and insulators on the basis of band theory and draw energy diagram. [1 + 1=2]
Question 16.
- Write any two differences between ideal and non-ideal solution.
- Calculate the osmotic pressure of M/25 solution of urea at 25°C temperature.
(R = 0.0821 L atm K-1mol-1) [1 + 1=2]
Question 17.
(A) Draw a labelled diagram of fuel cell. [1 + 1=2]
(B) Why the blue colour of copper sulphate solution disappears when zinc rod is dipped in it? Give reason.
![]()
Question 18.
Write any one difference between Ionisation and Hydrate isomerism in coordination compounds and give one example of each. [1 + 1=2]
Question 19.
(A) Write the chemical equation for preparation of Chloretone from Chloroform. [1 + 1=2]
(B) Explain the reason why alkylhalide reacts with KCN forms alkyl cyanide while when reacts with AgCN forms alkyl isocvanide.
Question 20.
Calculate electrode potential of copper electrode in 0.1 M copper ion solution. If standard electrode potential of copper is 0.34 V(log10 = 1) [1 + ½ + ½=2]
Question 21.
(A) Explain why fluoroacetic acid is stronger acidic than iodoacetic acid? [1 + 1=2]
(B) Write any one chemical reaction to differentiate between aldehyde and ketone.
OR
(A) Explain why formaldehyde is more reactive than acetone?
(B) Write any one chemical reaction to differentiate between formaldehyde and acetaldehyde.
Question 22.
Write the monomers of following polymers. [1 + 1=2]
(A) Nylon-6,6
(B) Terylene
Question 23.
(A) Write any one difference between enantiomer and diastereomer. [1 + 1=2]
(B) Give reason why the melting point of cis but-2-ene-l, 4-dioic acid is less than its trans isomer.
Question 24.
Write any one difference between soap and detergent and write one example of each. [1 + 1=2]
![]()
SECTION – C
Question 25.
Read the given paragraph and write answers of the following questions. [1 + 1 + 1=3]
“In colloidal state the range of particles of dispersed phase is 103 pm to 106 pm. Colloidal solutions are of two types (1) Lyophilic (2) Lyophobic. Stability of colloidal sol is due to charge on colloid particles. Neutrilization of particles is called coagulation. Comparision of coagulating power of electrolyte is done on the basis of Hardy and Schultze’s rule. Protecting capacity of lyophilic colloid depends upon gold number.”
(A) Arrange the Cl–, P043-, CO32- ions in the increasing order of coagulating capacity for positive sol.
(B) Write any two differences between Lyophilic and Lyophobic colloids.
(C) What do you understand by Gold number?
Question 26.
Read the given paragraph and write answers of the following questions. [1 + 1 + 1=3]
“Glucose is a carbohydrate found in huge amount in nature. It exists in both free and combined states. In free state, it is present in ripe grapes, honey, and sweet fruits. In human blood approximately 0.1 % of glucose is present. In combined state, it is present in form of disaccharide and polysaccharide. Sucrose is a disaccharide having glucose and fructose in equal amount it is a type of oligosaccharide. Starch is a polysaccharide having glucose but it is tasteless. Glucose is reducing sugar, due to this it gives test with Tollen’s and Fehling reagent.”
(A) Give the type of carbohydrate which on hydrolysis give two or maximum ten monosaccharide units.
(B) Write the name of functional group present in glucose due to which it gives test with Tollen’s reagent.
(C) What do we call the carbohydrates present in nature which are not sweet in taste? Give one example.
Question 27.
(A) Explain the weak monoacidic basic nature or urea by giving one chemical reaction. [1 + 1 + 1=3]
(B) Identify and write chemical formula of A and B in the following sequence of chemical Reaction.
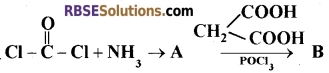
(C) Draw the resonating structures of nitrobenzene.
OR
(A) Aniline is less basic than alkylamine. Explain by giving one chemical reaction.
(B) Identify and write chemical formula of A and B in the following sequence of chemical reaction.
![]()
(C) Draw the resonating structures of aniline.
![]()
SECTION-D
Question 28.
(A). Write definition of zero order reaction. Write unit of its rate constant. [1 + 1 + 2=4]
(B). Write Arrhenius equation.
(C). The rate constant of one concentration is 60 s-1. How much time will be required to decompose 1/10th part of its initial concentration.
OR
(A) Write definition of activation energy. Write its S.I. unit.
(B) Write an equation which shows relation between rate constant (k0), initial concen-tration (a) and half life period (t1/2) for zero order reaction.
(C) Calculate rate constant for a zero order reaction in which 2 second time is re¬quired for change in concentration of reactant from 10 to 6 mol L-1.
Question 29.
(A) Draw structure of XeOF4
(B) Boiling point of PH3 is less than NH3 Give reason.
(C) Write an equation of chemical reaction of Cl2 with hot and concentrated NaOH.
(D) Halogens are strong oxidisin agents. Give reason. [1 + 1 + 1 + 1=4]
OR
(A) Draw structure of BrF3.
(B) Boiling point of HF is greater than HCl Give reason.
(C) Write an equation of chemical reaction of Zn with dilute HNO3
(D) Oxygen is a gas while sulphur is solid. Giver reason.
Question 30.
(A) What do you mean by Ziesel’s method?
(B) Explain why boiling point of ethanol is higher than methoxymethane?
(C) What happens when phenol reacts with concentrated nitric acid? Write chemical equation of reaction.
(D) Write IUPAC name of tertiary butyl alcohol. [1 + 1 + 1 + 1=4]
OR
(A) Write reaction of Williamson’s synthesis.
(B ) Explain the acidic nature of phenol.
(C) What happens when phenol reacts with phthalic anhydride in presence of concentrated H2SO4. Write chemical equation of the reaction.
(D) Write IUPAC name of orthocresol.
![]()
We hope the given RBSE Class 12 Chemistry Model Paper 2 English Medium will help you. If you have any query regarding RBSE Class 12 Chemistry Sample Paper 2 English Medium, drop a comment below and we will get back to you at the earliest.