Rajasthan Board RBSE Class 8 Science Notes Chapter 4 Chemical Reactions
Chemical Reactions:
Such a process in which organization and chemical properties of substances is changed are called chemical reactions.
Substances which takes part in reaction are called reactants and substances formed after reaction are called products. Examples- photosynthesis by plants, digestion of food in our body, out coming of bubbles while juice of lemon is put into soda water. Conversion of cut apple into brown color, rusting etc. are examples of chemical reactions.
- During a chemical reactions, bond among substances are formed and break.
- When iron and sulphur powder are taken in a China dish and heated, they form a new substances iron sulphide of a new color.
- In a chemical reaction, reactant are written on left hand side and products are written on right hand side.
Properties Of Chemical Reaction:
Iron reacts with oxygen and moisture present in the air forms the iron oxide (rust) in a chemical reaction.
- Production of gas: When fuel like petrol, kerosene etc are burnt then carbon present in them reacts with air and forms carbon dioxide (CO2) in the same manner CO2 is produced when coal is burnt.
C + O → CO2 (gas)
When sulphuric acid is poured slowly in a test tube in granulated zinc, hydrogen gas is produced. If a burning matchstick is taken at the mouth of the test tube it burns with an explosion. - Change in colour: Colour of cut apple is changed because iron present in apple reacts with oxygen present in the air and iron oxides forms.
- Change of heat: When ammonium chloride is put in water it becomes cold.
- Dissolution: In hard water, soap does not form foams but produces white undissolved substance.
Types of Chemical Reactions:
- Addition reaction: When two or more than two elements or compounds combine and produces a new product (compound) is called an addition reaction.
- Iron (Fe) + Oxygen (O2 from air) → Iron oxide (Fe3O4)
- Dissociation or decomposition of reactions:
Powder of lime
![]()
When lime powder is heated, a gas is produced when it is allowed to blow in lime water, it turns milky. This gas is CO2. A reaction in which a reactant decomposes and forms two or more products is called decomposition reaction.
- Displacement reaction: Those reactions in which less reactive element of compound is displaced by more reactive element is called displacement reaction.
Iron + Copper sulphate → Ferrous sulphate + Copper
Fe + CuSO4 → FeSO4 + Cu
When iron nails are put into 5 ml water solution of copper, after some time they will be coated with brown coat. This coat is of copper metal. The colour of solution also convert from blue to green. Here copper is displaced by iron which forms a coat on nails. Iron is more reactive than copper. - Redox reaction or oxidation: When powder of copper is heated in a china dish after sometime a black layer is coated on powdered copper. This black substance is copper oxide which is formed due to the combination of copper and oxygen copper.
Copper + Oxygen → Copper oxide
(from air) (inversion of oxygen
Cu +O2 → CuO
In this reaction copper is oxidised into copper oxide.
\(\begin{array}{l}{\text { Methane } \frac{\text { Heat }}{600^{\circ} \mathrm{C}} \text { Carbon }+\text { Hydrogen (release of hydrogen) }} \\ {\mathrm{CH}_{4} \quad \mathrm{C}+\mathrm{H}_{2}}\end{array}\)
Those reactions in which oxygen is used and hydrogen is released are called oxidation reaction. Oxidation and reduction are supplementary of each other. When one substance is oxidized another is redox are called as oxidation-reduction reactions. - Reduction reaction:
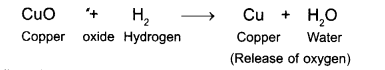
When hydrogen gas is flown over heated copper oxide copper and water are formed.
- Neutralization reaction: When any acid (HCl) and alkali (NAOH) are mixed in fixed amount and volume then salt and
- water are produced are called neutralization reaction.
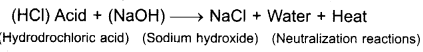
- Endothermic and exothermic reactions: Those reactions in which heat is absorbed are called endothermic reactions while those reactions in which heat is released are called exothermic reactions. Take some water in a beaker note its then temperature. When a pinch of very fine powder of potassium nitrate (KNO3) is mixed in water and temperature is taken again, it decreases. It is an endothermic reaction. On the other hand when same activity is repeated when (NaOH) sodium hydroxide temperature increases. Its an exothermic reaction.