Rajasthan Board RBSE Class 9 Science Notes Chapter 2 Structure of Matter and Molecule
- We see around us a large variety of things having different shapes, sizes and structures. Everything in the universe is made up of material or matter. Our body, the food we eat, air we breathe, clothes, plants, animals, stars, water, sand etc. all are matter. All these things have certain mass and occupy some space. Anything that has mass and occupies space is known as matter.
- Ancient Indian and Greek philosophers had classified matter into five groups, known as ‘Panch Tatva’ or five basic elements – air, water, earth, fire and sky. Modern scientists classify matter in two ways:
- Based on their physical properties as solid, liquid and gas.
- Based on chemical properties as element, mixture and compound.
Matter
- Particles of matter are very small in size, beyond our imagination.
- The particles remain continuously in motion. Therefore, the particles have kinetic energy.
- Particles of matter have force of attraction acting between them (known as inter-molecular force).
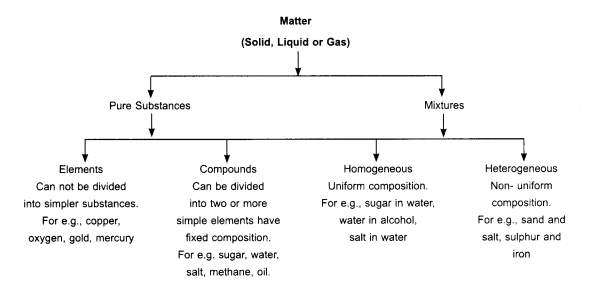
Due to presence of components in matter, it is divided into two types:
- Pure matter: Consist of only one type of component.
For example- Iron, Gold, Water etc. - Impure matter: Consist of one or more than one type of components.
For example- Cold drinks, Soil, Air etc.
Matter exists in three states:
solid, liquid and gas. Beside these, two other states of matter also exist. They are-Plasma state , Bose – Einstein Condensate-(BEC)
- Solid: Solids have a definite shape, distinct boundaries and fixed volume.
- Liquid: Liquids do not have definite shape, but have fixed volume. Liquids can flow and change their shape, so they are not rigid. These are also called as fluids.
- Gas: Gases have neither a fixed shape, nor a fixed volume. Gases are highly compressible.
- The force of attraction between the particles is maximum in solids, minimum in gases. Particles in solids are most orderly arranged. In liquids, the layers of particles can slip or slide over each other. In gases, there is no order and the particles are free to move about randomly.
Kanad Theory:
- Around 500 B.C an Indian philosopher Maharishi Kanad postulated that if we go on dividing matter (padarth) a time comes when we come across the smallest particles beyond which further division is not possible. He named these particles, Parmanu.
- Around the same era, ancient Greek philosopher. Democritus called these smallest particles atoms (meaning indivisible). By the end of the eighteenth century, scientists recognized the difference between elements and compounds. Antoine L. Lavoisier laid the foundation of chemical science by establishing two important laws of chemical combination.
Atoms:
- Atoms are very small. More than millions of atoms when stacked would make a layer barely as thick as a sheet of paper.
- Dalton was the first scientist to use the symbols for elements in a very specific sense. When he used a symbol for an element he also meant a definite quantity of that element, that is, one atom of that element. Berzillus suggested that the symbols of elements be made from one or two letters of the name of the element.
- Atoms of most elements do not exist freely. Atoms form molecules and ions. These molecules or ions aggregate in large numbers to form the matter.
Molecule:
- A molecule is a group of two or more atoms that are chemically bonded together. A molecule is the smallest particle of an element or a compound that is capable of an independent existence and shows all the properties of that substance.
- The molecules of an element are constituted by the same type of atoms. Molecules of argon (Ar), helium (He) etc. are made up of only one atom of that element.
- These are mono atomic. Most of non-metals, for example, oxygen consist of two atoms of oxygen and hence it is known as a diatomic molecule, 02, Ozone 03 is a tri atomic molecule. The number of atoms constituting a molecule is called its atomicity.
Elements:
- A pure substance which cannot be subdivided into two or more simpler substances by any chemical means is called an element.
- Elements are also divided into three groups: metals, non-metals and metalloids.
Compounds:
A compound is a substance composed of two or more elements, chemically combined with each other in a fixed proportion. The components can be separated only by chemical or electro-chemical methods, and cannot be separated by simple physical methods.
Mixture:
- Mixtures are constituted by more than one kind of pure form of matter known as a substance. We can have different kinds of mixtures.
- A mixture which has a uniform composition throughout is called homogeneous mixture or solution e.g. sugar dissolved in water.
- A mixture which contains two or more types of distinct particles and has a non-uniform composition is called heterogeneous mixture, e.g. mixture of salt and sulfur or oil and water
Physical and Chemical Change
- Physical change: A change which changes only some specific property of matter, without any change in the composition of its molecules and its chemical nature, is called physical change.
For example: Melting of ice. On heating, ice changes into water but their chemical components are same and their molecular formula is also same i.e.
H2O (S) → H2O (I) - Chemical change: A change which changes all the specific properties of a material, bringing change in its molecular composition and producing a new substance, is called chemical change.
For example: Carbon burnt in presence of oxygen produces carbon dioxide. Their states and chemical composition also change.

States of matter and their effects
Effect of Temperature:
- On increasing the temperature, kinetic energy of the particles increases, so they vibrate and move with greater speed. Increased energy overcomes the force of attraction between the particles and their order is disturbed. Thus, on heating, a solid changes into liquid and a liquid is changed into gas at certain fixed temperature. On decreasing the temperature, the change takes place in the reverse order.
![]()
- The temperature at which a solid melts, i.e. changes into liquid state at the atmospheric pressure is called its melting point. Process of melting is also known as fusion. Ice melts at 0°C or 273 K.
- The temperature at which a liquid changes into gaseous state at the atmospheric pressure is called its boiling point. Water boils at 100°C or 373 K.
- Melting point of a solid or boiling point of a liquid gives an indication of the strength of force of attraction between its particles.
- At its melting point a solid starts melting, but its temperature does not rise till whole of it is melted, even though heat energy is continuously supplied. This heat energy is absorbed by the solid without showing any rise in temperature, and is used up in overcoming the forces of attraction between the particles and making them free to move apart. It is known as latent heat (latent means hidden).
- Amount of heat energy required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion of that solid.
- Similarly, at its boiling point a liquid starts changing into vapours, but its temperature does not rise till whole of it changes into gaseous form, even though heat energy is continuously supplied. This heat energy is absorbed by the liquid without showing any rise in temperature, and is used up in overcoming the forces of attraction between the particles and making them free to move apart.
- Amount of heat energy required to change 1 kg of a liquid into gas at atmospheric pressure at its boiling point is known as the latent heat of vaporisation of that liquid.
- Changing’of gas into liquid is known as condensation. Changing of liquid into solid is known as freezing or solidification. Latent heat is given out during the process of condensation and freezing.
- There are some substances which change directly from solid state to gaseous state on heating and from gaseous state to solid state on cooling, without changing into intermediate liquid state. Such substances are called sublime and the process is called sublimation.
Effect of Pressure:
By increasing or decreasing the pressure, the state of matter can be changed. On applying pressure, particles of the gas come closer and closer. A stage is reached, when it is converted into liquid form. Carbon dioxide under high pressure changes into solid form directly (known as dry ice), and on reducing the pressure it gets converted to gaseous state without converting into liquid state.
Separation of Components of a mixture:
Components of a mixture can be separated by simple physical methods like handpicking, sieving, winnowing, sedimentation, decantation, filtration, evaporation, sublimation etc. Some special techniques are also used, like fractional distillation, crystallisation, centrifugation and chromatography, depending upon the nature of constituents.
Filtration:
- This is a method of separating solid constituents from the liquid in a heterogeneous mixture.
- In the process of filtration solid is collected on the filter paper and liquid is formed in the form of filtrate. For example, sand is separated from sand water.
Crystallisation:
- Crystallisation is a process that separates a pure solid in the form of its crystals, from a solution. Crystals are the purest form of a substance and have definite geometrical shapes.
- Impure crystalline solids are purified by this method. In this method, the impure solid is dissolved in a minimum amount of water, to form solution which is filtered to remove in soluble impurities. Then, the clear solution is heated gently on a water bath, till a concentrated solution is obtained. The solution is allowed to cool slowly, by leaving it undisturbed for some time. Crystals of pure solid are formed, while impurities remain dissolved in solution. This method is employed for purifying copper sulphate, alum (phitkari), sugar, common salt, etc.
Sublimation:
The process by which a solid directly changes into the gaseous state on heating, without changing into the intermediate liquid state, and the gaseous state directly changes into the solid state on cooling, is known as sublimation. Camphor and ammonium chloride sublime on heating.
Differential Extraction:
Immiscible liquids in a mixture form different layers depending on their densities, when allowed to stand undisturbed for some time. Each layer can be separately taken out, by using a separating funnel. Thus, the components of a mixture of two immiscible liquids is separated by using a separating funnel.
Distillation:
When the two liquids in the mixture are such that they boil without decomposition or chemical reaction and their boiling points have sufficient difference, more than 25°C, then they can be separated by simple distillation.
Fractional Distillation:
- If the difference in boiling points of the constituent liquids in mixture is less than 25°C, the method of fractional distillation is used to separate them. Fractional distillation method is same as simple distillation, except that in this technique a fractionating column is fitted in between the distillation flask and condenser. It makes the apparatus more efficient.
- Fractionating column is a wide tube packed with glass beads, which provides larger surface area for cooling. Vapours of liquid having higher boiling point get condensed on the surface of these beads, while the vapours of lower boiling point liquid pass on upwards. Length of the column also increases the efficiency of the process. For example: In an oil refinery, the different fractions of petroleum products are separated by fractional distillation of crude petroleum oil.